This document provides an overview of solar cell fundamentals:
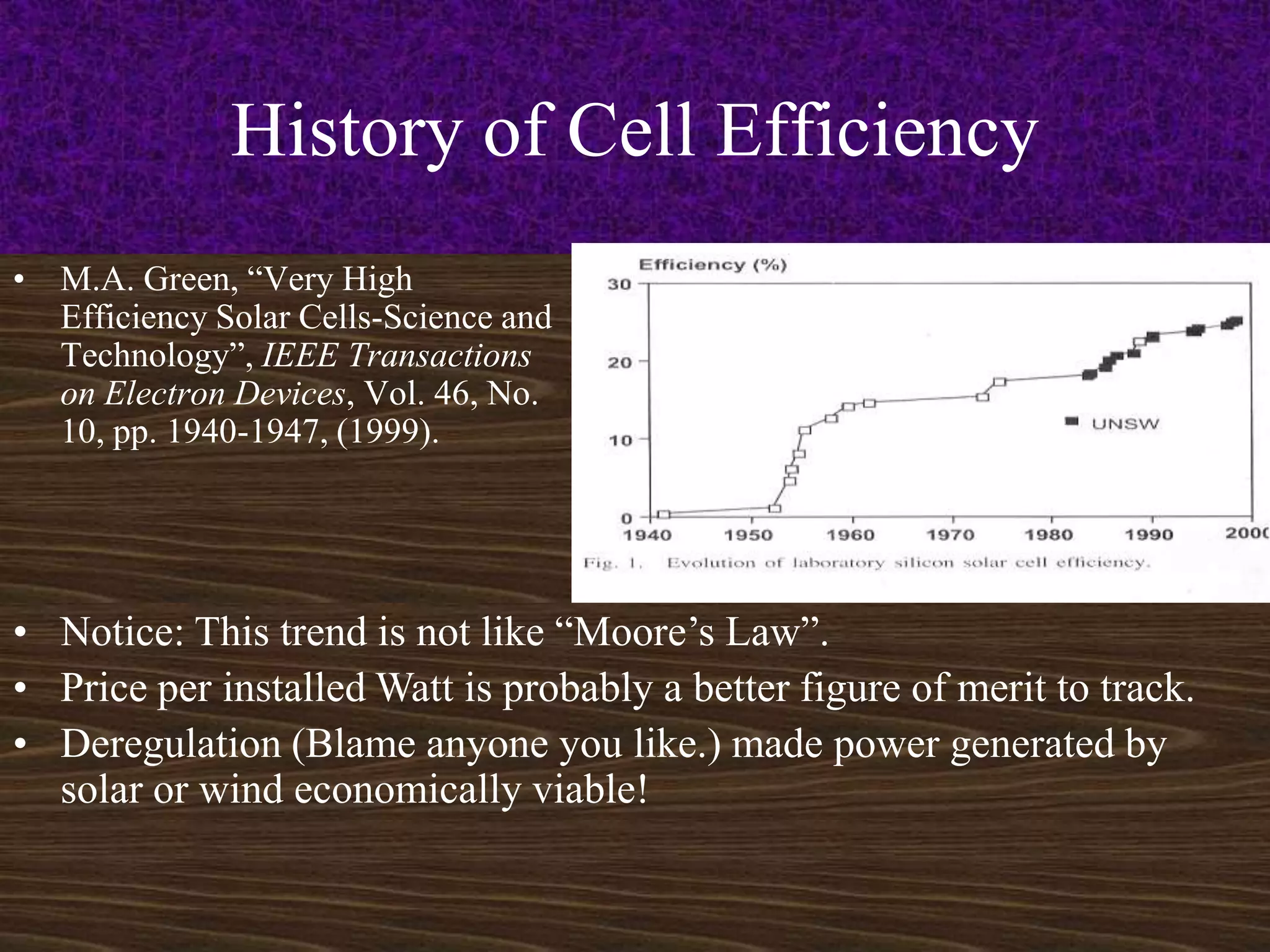
1. The history of increasing solar cell efficiency over time, though not following Moore's Law of consistent doubling every couple years like computer chips. Price per watt installed is a better metric.
2. What a solar cell is and how it works, converting sunlight directly to DC electricity through semiconductors like silicon.
3. The various uses of solar energy from powering homes and buildings to applications in planes, boats, and other vehicles.