1. Preterm prelabour rupture of membranes complicates approximately 3% of pregnancies, causing about one-third of all preterm deliveries. It is associated with high neonatal mortality and both short- and long-term severe morbidity.
2. Expectant management through latency period monitoring remains the standard of care in the absence of infection or other complications, as prematurity confers most of the fetal and neonatal risk.
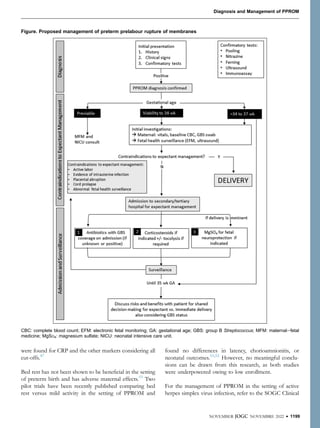
3. The guideline provides recommendations for diagnosing preterm prelabour rupture of membranes using history, physical exam including sterile speculum exam, and confirmation with multiple conventional tests or commercial tests for equivocal cases.




![of infection or rupture of the membranes and thus is not
routinely performed.
In order to improve the diagnostic accuracy of PPROM
and avoid undertreatment or overtreatment, several diag-
nostic tests have been developed in the last few decades.24
Three systematic reviews evaluating novel methods of
diagnosis of PPROM include non-conventional “com-
mercial” immunoassay tests (for insulin-like growth factor-
binding protein-1 [IGFBP-1] and placental alpha
microglobulin-1 [PAMG-1]).26e28
Compared with nitra-
zine or ferning test alone, both immunoassay tests showed
increased accuracy but there was no statistical difference in
equivocal cases.26,27
The comparison between the 3 tests
showed a higher accuracy for PAMG-1.28
These system-
atic reviews should be considered with caution because the
sample sizes of the included studies were small, the studies
were all observational, and there was no reliable gold
standard test against which the commercial tests were
compared. A more recent prospective multicentre study
compared the performance of PAMG-1 with the intra-
amniotic infusion of indigo carmine dye in 140 patients
with unknown membrane status. The results showed that
the PAMG-1 test performed as well as the gold standard
with a sensitivity of 100% (95% CI 0.95e0.99), specificity
of 99.1% (95% CI 0.82e0.99), positive predictive value of
96.3% (95% CI 0.82e0.99), and negative predictive value
of 100% (95% CI 0.97e1.0).29
Despite high costs, commercial tests should be considered
as primary diagnostic tests in rural and remote where no
other diagnostic options are available or feasible.
Table 1. Performance of current available diagnostic tests for preterm prelabour rupture of membranes
Test N Sensitivity (%) Specificity (%) PPV (%) NPV (%) Accuracy (%)
Gold standard
Intraamniotic dye injection 18 d d d d d
Clinical
Clinical history 100 90 89 88 90 89
Conventional tests
Nitrazine pH125e129
618 84-97 16-94 84-99 68-98 56-93
Arborization 642 51-96 71-100 61-100 73-91 63-96
Ultrasound
AFI 151 94 91 d d 92
Combined testing
History þ arborization þ nitrazine 100 91 95 96 91 93
History þ 2/3 of pooling, arborization, nitrazine 69 86 37 78 50 73
History þ arborization þ nitrazine þ AFI 167 89-94 95-100 95-100 88-98 91-98
Commercial immunoassays
PAMG-1a128e133
598 90-100 93-100 98-100 86-100 94-100
IGFBP-1b
513 90-100 98-99 97-98 90-100 93-99
Other vaginal fluid markers
Prolactin (cut-off 20-30 mIU/mL)134,135
170 76-95 70-78 72-84 75-93 87
AFP (cut-off 35.9-125 mg/L) 180 94-100 94-100 94-100 94-100 d
b-hCG (cut-off 25-80 mIU/mL) 275 79-93 72-96 75-95 82-84 88
Lactate (cut-off 4.5 mmol/L) 200 86 92 92 87 d
Creatinine (cut-off 0.12-0.6 mg/dL) 339 89-100 90-100 89-100 89-100 d
Urea (cut-off 6.7-12 mg/dL)136,137
339 88-100 91-100 91-100 88-100 d
Haptoglobin (cut-off 94.5 mg/dL) 60 80 80 d d d
Thyroid hormone 60
Total T4 (cut-off 0.866 mg/dL) 83 60 68 78 d
Free T4 (cut-off 0.079 ng/L) 90 70 75 88 d
a
AmniSure ROM is the trade name of the commercial test for detecting PAMG-1.
b
Actim PROM is the trade name of the commercial test for detecting IGFBP-1.
AFI: amniotic fluid index; AFP: a-fetoprotein; ß-hCG: beta subunit of human chorionic gonadotropin; IGFBP-1: insulin-like growth factor binding protein-1; PAMG-1:
placental alpha microglobulin-1; T4: thyroxine.
NOVEMBER JOGC NOVEMBRE 2022 l 1197
Diagnosis and Management of PPROM](https://image.slidesharecdn.com/sogc2022ppromcpg430-231015191741-81a462c0/85/SOGC-2022-PPROM-cpg430-pdf-5-320.jpg)




![PROMEXIL trial have highlighted some increased risks.
One study addressed the neurodevelopmental outcomes
of children at 2 years of age and found more neuro-
developmental problems after expectant management than
after early induction of labour.98
The other study sug-
gested that patients with GBS colonization would benefit
the most from immediate delivery owing to a significantly
lower incidence of early onset neonatal sepsis seen with
intervention (15.2% in expectant management vs. 1.8% in
immediate delivery; RR 0.1 [95% CI 0.01e0.84]). In the
subgroup of GBS-negative patients, the risk of early onset
neonatal sepsis was lower and not significantly different
between expectant management and immediate delivery.99
These results are in contrast with those of a subgroup
analysis of PPROMT, which did not find differences in
early onset neonatal sepsis between GBS-positive patients
and managed expectantly and those who delivered
immediately (4% expectant management vs. 3% immediate
delivery; RR 0.9 [95% CI 0.2e4.5]).97
However, a recent
systematic review, which included both PPROMT and
PROMEXIL trials, confirmed the clinical and cost effec-
tiveness of immediate delivery over expectant management
in PPROM between 34 and 36 weeks for patients with
GBS colonization.100
SUMMARY STATEMENT 3 AND
RECOMMENDATION 16
PPROM in Patients with Cerclage
Although PPROM is a relatively frequent complication of
cerclage, seen in as many as 38% of patients,101
manage-
ment of the cerclage after PPROM is controversial, with
no prospective studies yet completed on this topic.
The only prospective, randomized, multicentre trial to
date, comparing retention versus removal of the cerclage
after PPROM between 220/7
and 326/7
weeks, was
terminated at the second interim analysis because of
concern regarding low power.102
In their interim analysis,
the authors found no differences in latency period for
delivery, infection, or composite neonatal outcome, and a
nonestatistically significant lower incidence of complica-
tions related to infection was associated with immediate
removal of cerclage.
There are a few retrospective cohort studies, mostly with
small sample sizes, that found conflicting results regarding
the safety and efficacy of retaining a cerclage after
PPROM.103e106
Three literature reviews attempted to summarize available
data on this topic with inconsistent conclusions.
Giraldo-Isaza and Berghella suggested immediate cerclage
removal as the preferred approach owing to an increased
risk of maternal chorioamnionitis and neonatal mortality
from sepsis seen with retention,105
while Walsh et al.
concluded that, in the absence of large, well designed
studies, the decision to retain or remove cerclage in pa-
tients with PPROM should be done on an individualized
based on maternal clinical status.104
In a more recent meta-
analysis, Pergialiotis et al. stated that the current evidence
is insufficient to support the retention of cervical cerclage
after PPROM.103
SUMMARY STATEMENT 8 AND
RECOMMENDATION 17
PPROM at Previable Gestational Age
For information on the management of pregnancies at
borderline viability, refer to the SOGC Clinical Practice
Guideline No. 347, Obstetric management at borderline
viability.107
PPROM at previable gestational age complicates approx-
imately 0.1% of pregnancies and is associated with high
fetal and neonatal morbidity and mortality, ranging from
46% to 95%.108
Of note, iatrogenic previable PPROM
after an invasive obstetric procedure such as amniocentesis
or fetoscopy carries a better prognosis than spontaneous
previable PPROM owing to a higher incidence of resealing
of the amniotic sac, which has been reported to be as high
as 72% in these cases.7
To investigate the natural history of
previable PPROM, Linehan et al. completed a single centre
retrospective cohort study between 2007 and 2012, where
termination of pregnancy in the absence of maternal
compromise was unavailable. In their study, the mean
gestation age at PPROM was 18 weeks, the average latency
period for delivery was 13 days, and the mean gestational
age at delivery was 205
weeks. Overall mortality was 95%
(40/42), with only 10 infants born alive (23%; 10/42) and
2 surviving to discharge (5%).108
A study conducted be-
tween 2012 and 2017 that included a cohort of 192 pre-
viable PPROM patients (mean gestational age at PPROM
205
weeks), who were expectantly managed with a mean
delivery at 28.6 ± 5.1 weeks gestation, reported a neonatal
survival rate to hospital discharge of 54% with a 10% risk
of stillbirth.109
Two Canadian studies have also described
perinatal outcomes following previable PPROM. In a
cohort of 99 previable PPROM pregnancies between 2009
NOVEMBER JOGC NOVEMBRE 2022 l 1203
Diagnosis and Management of PPROM](https://image.slidesharecdn.com/sogc2022ppromcpg430-231015191741-81a462c0/85/SOGC-2022-PPROM-cpg430-pdf-11-320.jpg)



![67. Weston EJ, Pondo T, Lewis MM, et al. The burden of invasive early-onset
neonatal sepsis in the United States, 2005-2008. Pediatr Infect Dis J
2011;30:937e41.
68. Oh KJ, Romero R, Park JY, et al. The earlier the gestational age, the greater
the intensity of the intra-amniotic inflammatory response in women with
preterm premature rupture of membranes and amniotic fluid infection by
Ureaplasma species. J Perinat Med 2019;47:516e27.
69. Lal CV, Xu X, Jackson P, et al. Ureaplasma infection-mediated
release of matrix metalloproteinase-9 and PGP: a novel mechanism of
preterm rupture of membranes and chorioamnionitis. Pediatr Res
2017;81:75e9.
70. Navathe R, Schoen CN, Heidari P, et al. Azithromycin vs erythromycin for
the management of preterm premature rupture of membranes. Am J
Obstet Gynecol 2019;221:144.e1e8.
71. Pierson RC, Gordon SS, Haas DM. A retrospective comparison of
antibiotic regimens for preterm premature rupture of membranes. Obstet
Gynecol 2014;124:515e9.
72. Finneran MM, Smith DD, Buhimschi CS. Cost analysis of azithromycin
versus erythromycin in pregnancies complicated by preterm premature
rupture of membranes. Am J Perinatol 2019;36:105e10.
73. Finneran MM, Appiagyei A, Templin M, et al. Comparison of azithromycin
versus erythromycin for prolongation of latency in pregnancies complicated
by preterm premature rupture of membranes. Am J Perinatol
2017;34:1102e7.
74. Seaman RD, Kopkin RH, Turrentine MA. Erythromycin versus
azithromycin for treatment of preterm prelabor rupture of membranes: a
systematic review and meta-analysis. Am J Obstet Gynecol
2022;226:794e801.e1.
75. Wolf MF, Sgayer I, Miron D, et al. A novel extended prophylactic antibiotic
regimen in preterm pre-labor rupture of membranes: a randomized trial.
Int J Infect Dis 2020;96:254e9.
76. Money DM, Dobson S, Canadian Paediatric Society IDC. The prevention
of early-onset neonatal group B streptococcal disease. J Obstet Gynaecol
Can 2004;26:826e40.
77. Ronzoni S, Cobo T, D’Souza R, et al. Individualized treatment of
preterm PROM to prolong the latency period, reduce the rate of preterm
birth, and improve neonatal outcomes [e-pub ahead of print]. Am J Obstet
Gynecol https://doi.org/10.1016/j.ajog.2022.02.037, accessed July 25,
2022.
78. Skoll A, Boutin A, Bujold E, et al. No. 364-antenatal corticosteroid therapy
for improving neonatal outcomes. J Obstet Gynaecol Can
2018;40:1219e39.
79. Roberts D, Brown J, Medley N, et al. Antenatal corticosteroids for
accelerating fetal lung maturation for women at risk of preterm birth.
Cochrane Database Syst Rev 2017;3:CD004454.
80. Smith J, Murphy KE, McDonald SD, et al. Timing of antenatal
corticosteroids in relation to clinical indication [e-pub ahead of print]. Arch
Gynecol Obstet https://doi.org/10.1007/s00404-021-06362-7, accessed
July 25, 2022.
81. Magann EF, Haram K, Ounpraseuth S, et al. Use of antenatal
corticosteroids in special circumstances: a comprehensive review. Acta
Obstet Gynecol Scand 2017;96:395e409.
82. Fox NS, Gelber SE, Kalish RB, et al. Contemporary practice patterns and
beliefs regarding tocolysis among U.S. maternal-fetal medicine specialists.
Obstet Gynecol 2008;112:42e7.
83. Mackeen AD, Seibel-Seamon J, Muhammad J, et al. Tocolytics for preterm
premature rupture of membranes. Cochrane Database Syst Rev
2014;(2):CD007062.
84. Nijman TA, van Vliet EO, Naaktgeboren CA, et al. Nifedipine versus
placebo in the treatment of preterm prelabor rupture of membranes: a
randomized controlled trial: assessment of perinatal outcome by use of
tocolysis in early labor-APOSTEL IV trial. Eur J Obstet Gynecol Reprod
Biol 2016;205:79e84.
85. Lorthe E, Goffinet F, Marret S, et al. Tocolysis after preterm premature
rupture of membranes and neonatal outcome: a propensity-score analysis.
Am J Obstet Gynecol 2017;217:212.e1e212.e12.
86. Horton AL, Lai Y, Rouse DJ, et al. Effect of magnesium sulfate
administration for neuroprotection on latency in women with preterm
premature rupture of membranes. Am J Perinatol 2015;32:387e92.
87. Kamyar M, Manuck TA, Stoddard GJ, et al. Magnesium sulfate,
chorioamnionitis, and neurodevelopment after preterm birth. BJOG
2016;123:1161e6.
88. Magee LA, De Silva DA, Sawchuck D, et al. No. 376-magnesium sulphate
for fetal neuroprotection. J Obstet Gynaecol Can 2019;41:505e22.
89. Doyle LW, Crowther CA, Middleton P, et al. Magnesium sulphate for
women at risk of preterm birth for neuroprotection of the fetus. Cochrane
Database Syst Rev 2009;1:CD004661.
90. Wolf HT, Huusom LD, Henriksen TB, et al. Magnesium sulphate for fetal
neuroprotection at imminent risk for preterm delivery: a systematic review
with meta-analysis and trial sequential analysis. BJOG 2020;127:1180e8.
91. Clark EA, Varner M. Impact of preterm PROM and its complications on
long-term infant outcomes. Clin Obstet Gynecol 2011;54:358e69.
92. Bond DM, Middleton P, Levett KM, et al. Planned early birth versus
expectant management for women with preterm prelabour rupture of
membranes prior to 37 weeks’ gestation for improving pregnancy outcome.
Cochrane Database Syst Rev 2017;3:Cd004735.
93. Pasquier JC, Claris O, Rabilloud M, et al. Intentional early delivery versus
expectant management for preterm premature rupture of membranes at
28-32 weeks’ gestation: a multicentre randomized controlled trial
(MICADO STUDY). Eur J Obstet Gynecol Reprod Biol 2019;233:30e7.
94. Quist-Nelson J, de Ruigh AA, Seidler AL, et al. Immediate delivery
compared with expectant management in late preterm prelabor rupture of
membranes: an individual participant data meta-analysis. Obstet Gynecol
2018;131:269e79.
95. van der Ham DP, Nijhuis JG, Mol BW, et al. Induction of labour versus
expectant management in women with preterm prelabour rupture of
membranes between 34 and 37 weeks (the PPROMEXIL-trial). BMC
Pregnancy Childbirth 2007;7:11.
96. van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of
late-preterm premature rupture of membranes: the PPROMEXIL-2 trial.
Am J Obstet Gynecol 2012;207:276.e1e10.
97. Morris JM, Roberts CL, Bowen JR, et al. Immediate delivery compared
with expectant management after preterm pre-labour rupture of the
membranes close to term (PPROMT trial): a randomised controlled trial.
Lancet 2016;387:444e52.
98. van der Heyden JL, Willekes C, van Baar AL, et al. Behavioural and
neurodevelopmental outcome of 2-year-old children after preterm
premature rupture of membranes: follow-up of a randomised clinical trial
comparing induction of labour and expectant management. Eur J Obstet
Gynecol Reprod Biol 2015;194:17e23.
99. Tajik P, van der Ham DP, Zafarmand MH, et al. Using vaginal group B
Streptococcus colonisation in women with preterm premature rupture of
membranes to guide the decision for immediate delivery: a secondary
analysis of the PPROMEXIL trials. BJOG 2014;121:1263e72; discussion
73.
100. Evidence review for timing of delivery to reduce the risk of early-onset
neonatal infection: neonatal infection: antibiotics for prevention and
treatment. London: NICE Evidence Reviews Collection; 2021. Available at:
https://www.ncbi.nlm.nih.gov/books/NBK571215/. Accessed on July 25,
2022.
101. Treadwell MC, Bronsteen RA, Bottoms SF. Prognostic factors and
complication rates for cervical cerclage: a review of 482 cases. Am J Obstet
Gynecol 1991;165:555e8.
102. Galyean A, Garite TJ, Maurel K, et al. Removal versus retention of cerclage
in preterm premature rupture of membranes: a randomized controlled trial.
Am J Obstet Gynecol 2014;211:399.e1e7.
NOVEMBER JOGC NOVEMBRE 2022 l 1207
Diagnosis and Management of PPROM](https://image.slidesharecdn.com/sogc2022ppromcpg430-231015191741-81a462c0/85/SOGC-2022-PPROM-cpg430-pdf-15-320.jpg)

