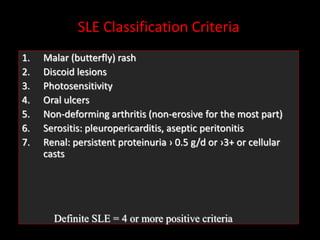
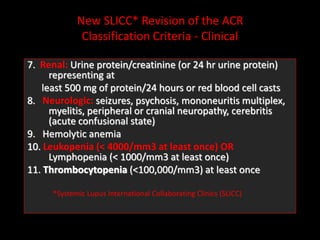
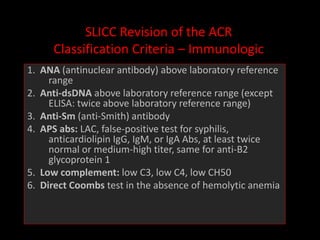
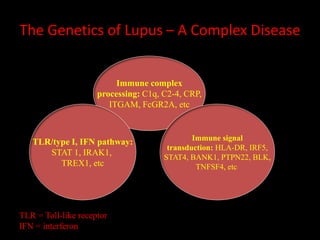
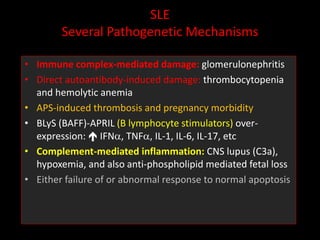
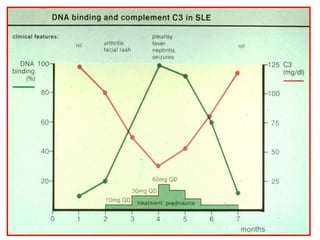
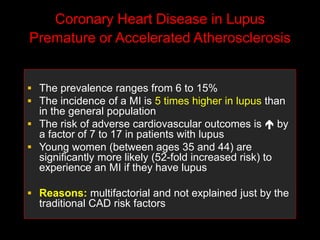
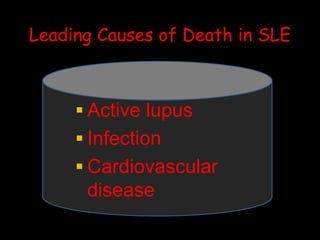
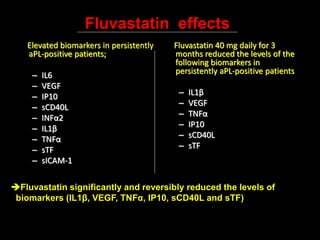
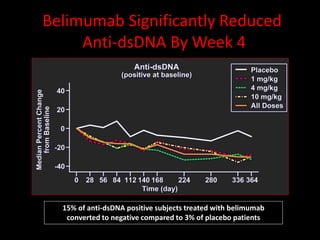
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease characterized by abnormal autoantibodies and polyclonal B-cell activation. SLE severity ranges from mild skin and joint involvement to severe renal, lung, and central nervous system disease. New classification criteria require at least 4 clinical and/or immunologic criteria, including nephritis by biopsy. Genetic susceptibility involves genes related to immune complex processing, interferon pathway regulation, and immune signal transduction. Treatment focuses on hydroxychloroquine, corticosteroids, immunosuppressants, and the biologic belimumab to reduce disease activity and flares while minimizing medications. Targeted therapies aim to block B-cells,