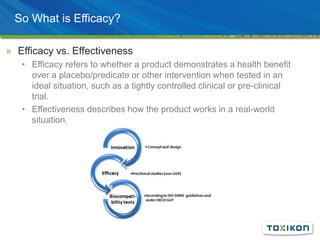
Regulatory Submission: Applying GLP in Surgical Efficacy Studies discusses the application of Good Laboratory Practices (GLP) standards to preclinical surgical efficacy studies. It notes that while safety data must always adhere to GLP, efficacy studies are sometimes conducted non-GLP. The document reviews key aspects of GLP compliance including facilities, equipment, personnel roles, test article handling, protocols, standard operating procedures, record keeping and reporting to ensure data integrity and reproducibility. Pilot studies, experimental design considerations and the value of efficacy data for submissions and product differentiation are also discussed.








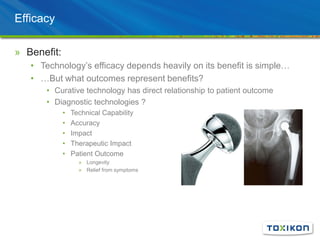




![FDA Device Classification: Risk-Based Approach
» • Class 1: Common, low-risk devices
» General controls
» Most exempt from pre-market submission
» • Class 2: More complex, higher risk
» Special controls
» Pre-market notification [510(k)]
» • Class 3: Most complex, highest risk
» (Devices which support or sustain human life; devices which
pose potential unreasonable risk of illness or injury)
» Comprehensive data needed
» Pre-market application [PMA]](https://image.slidesharecdn.com/toxikonboston-140401115309-phpapp01/85/Regulatory-submission-Applying-GLP-in-surgical-efficacy-studies-18-320.jpg)