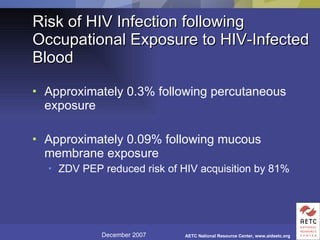
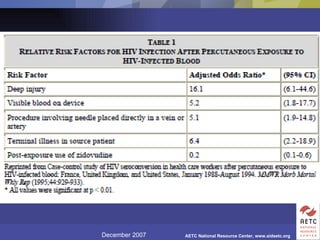
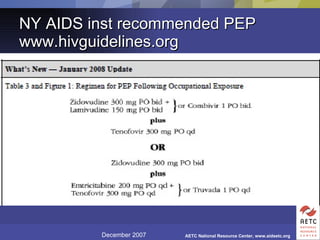
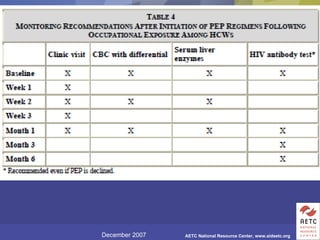
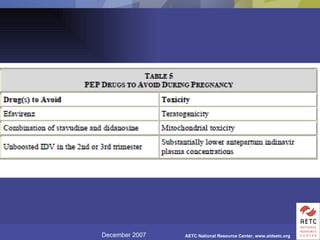
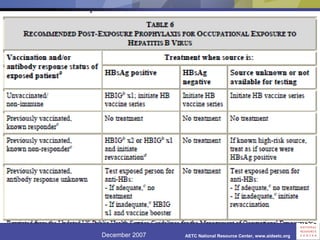
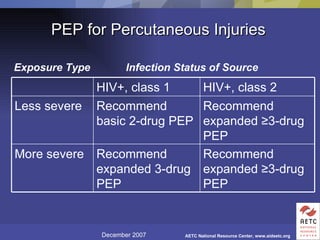
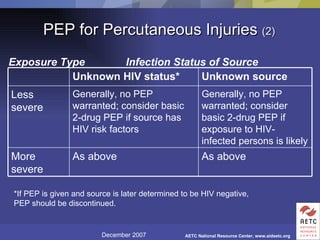
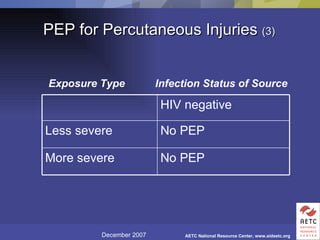
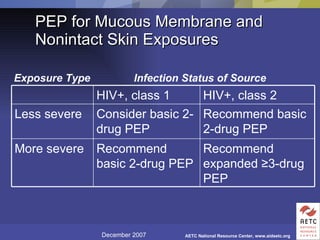
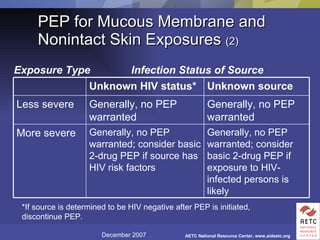
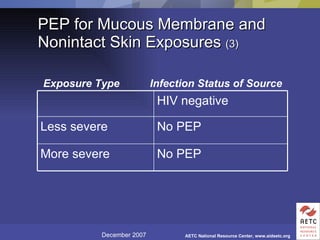
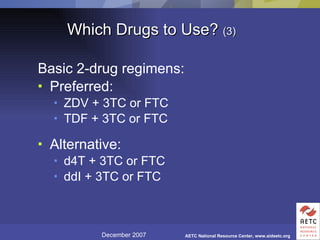
The document provides guidelines for managing occupational exposures to HIV, including recommendations for postexposure prophylaxis (PEP). It outlines when PEP is indicated, how to initiate it within 36 hours of exposure, and selecting an appropriate drug regimen for 4 weeks. It also discusses evaluating the exposed individual within 72 hours and considering source patient testing to help determine if PEP should continue or be discontinued.