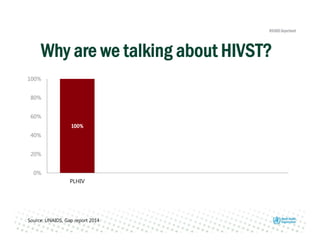
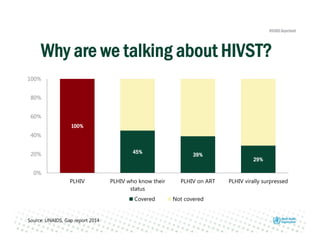
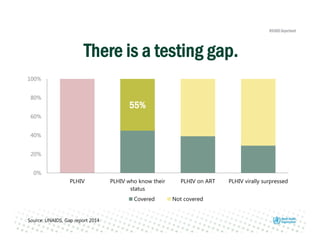
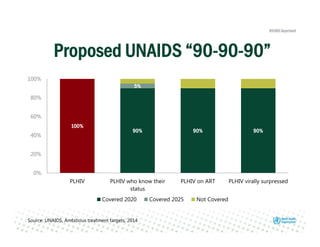
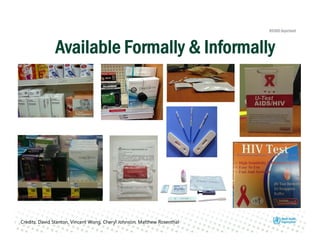
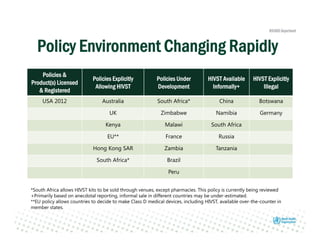
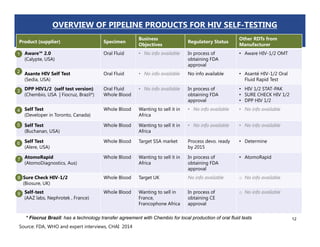
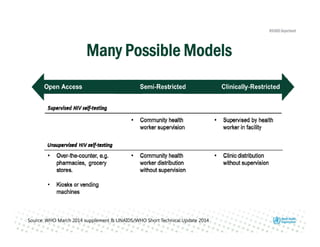
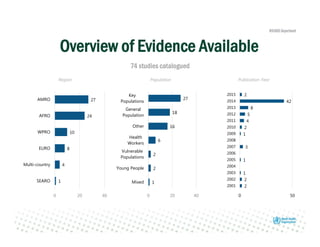
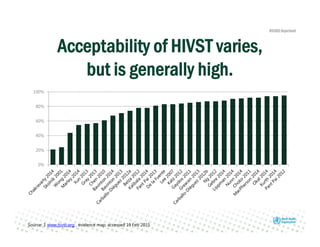
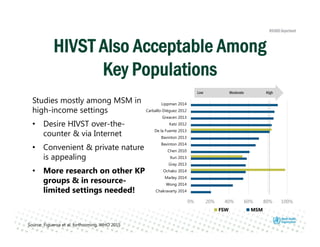
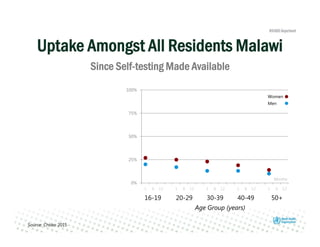
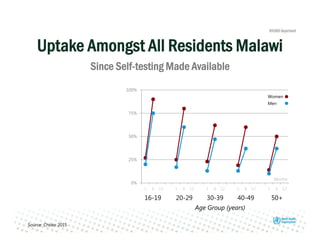
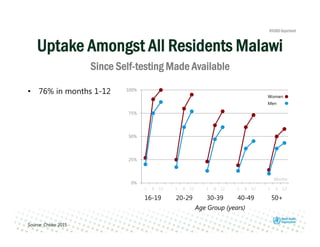
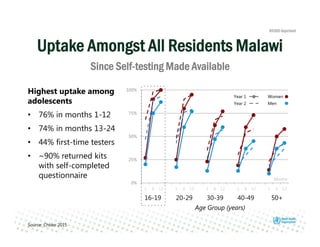
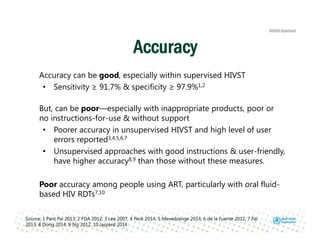
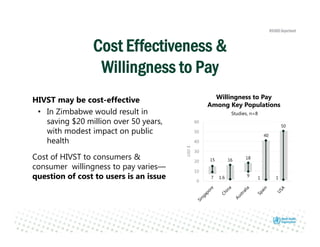
Rachel Baggaley presented an overview of HIV self-testing at the Bill and Melinda Gates Foundation meeting. There is a large testing gap, with only 45% of people living with HIV knowing their status. HIV self-testing could help close this gap by making testing more convenient and private. Several HIV self-testing products are in development using oral fluid or whole blood samples. Early evidence shows high acceptability of HIV self-testing, though accuracy can vary depending on how it is administered. Linkage to care also seems promising when self-testing is coupled with support services. More research is still needed on self-testing among key populations and in resource-limited settings.