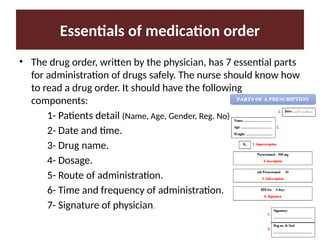
Discuss the terminologies related to pharmacology
Discuss the history of pharmacology briefly
Identify the purposes of medication
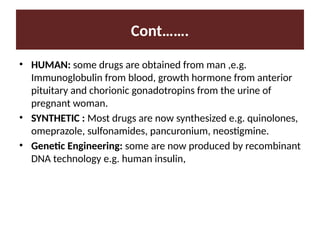
Identify the source of medication
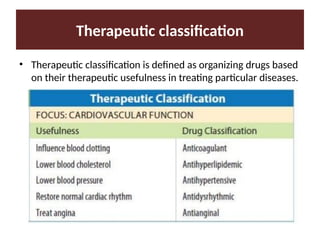
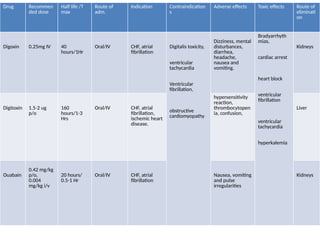
Discuss the classification of drugs
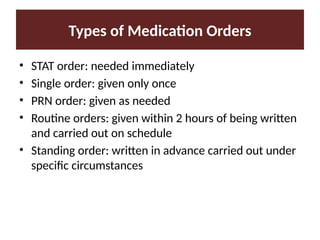
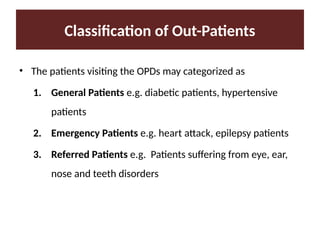
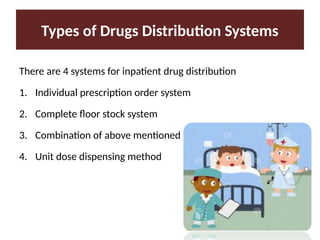
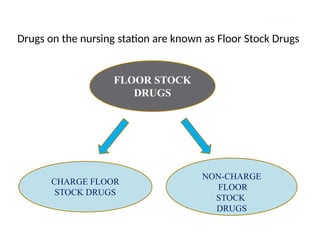
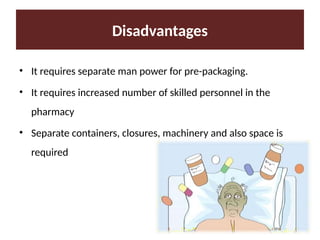
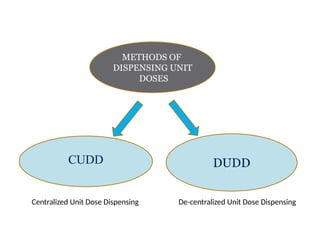
Describe the three type of drug supply system.
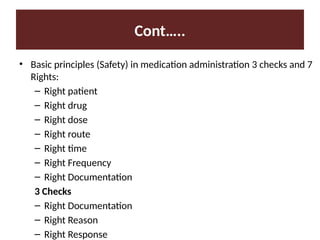
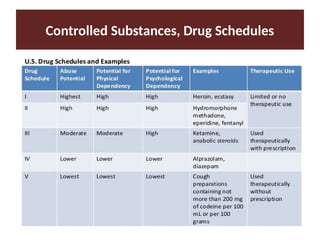
Discuss the drugs standards and legislation.
Identify resource to collect and utilize drug information.
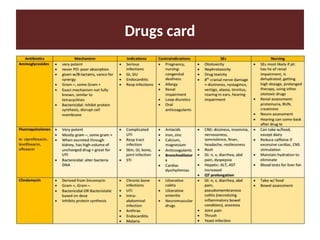
Learn to prepare drugs cards