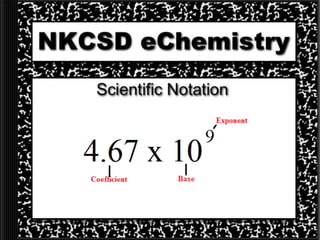
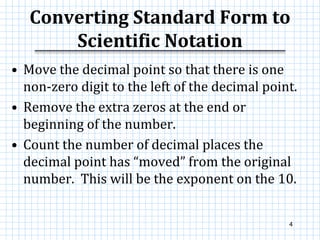
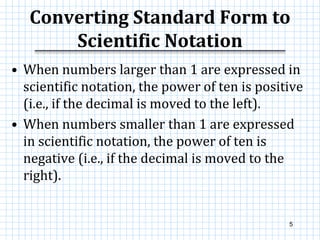
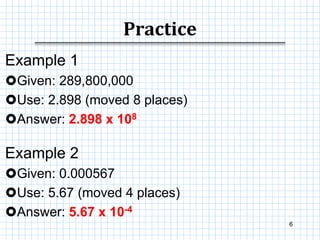
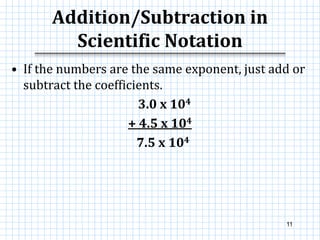
This document provides information on scientific notation and how to perform calculations using numbers expressed in scientific notation. It defines scientific notation as expressing numbers in the form of N x 10x, where N is the coefficient and x is the exponent. It explains how to convert between standard and scientific notation by moving the decimal point and counting places. The document also outlines how to perform addition, subtraction, multiplication and division when numbers are in scientific notation by manipulating the coefficients and exponents.