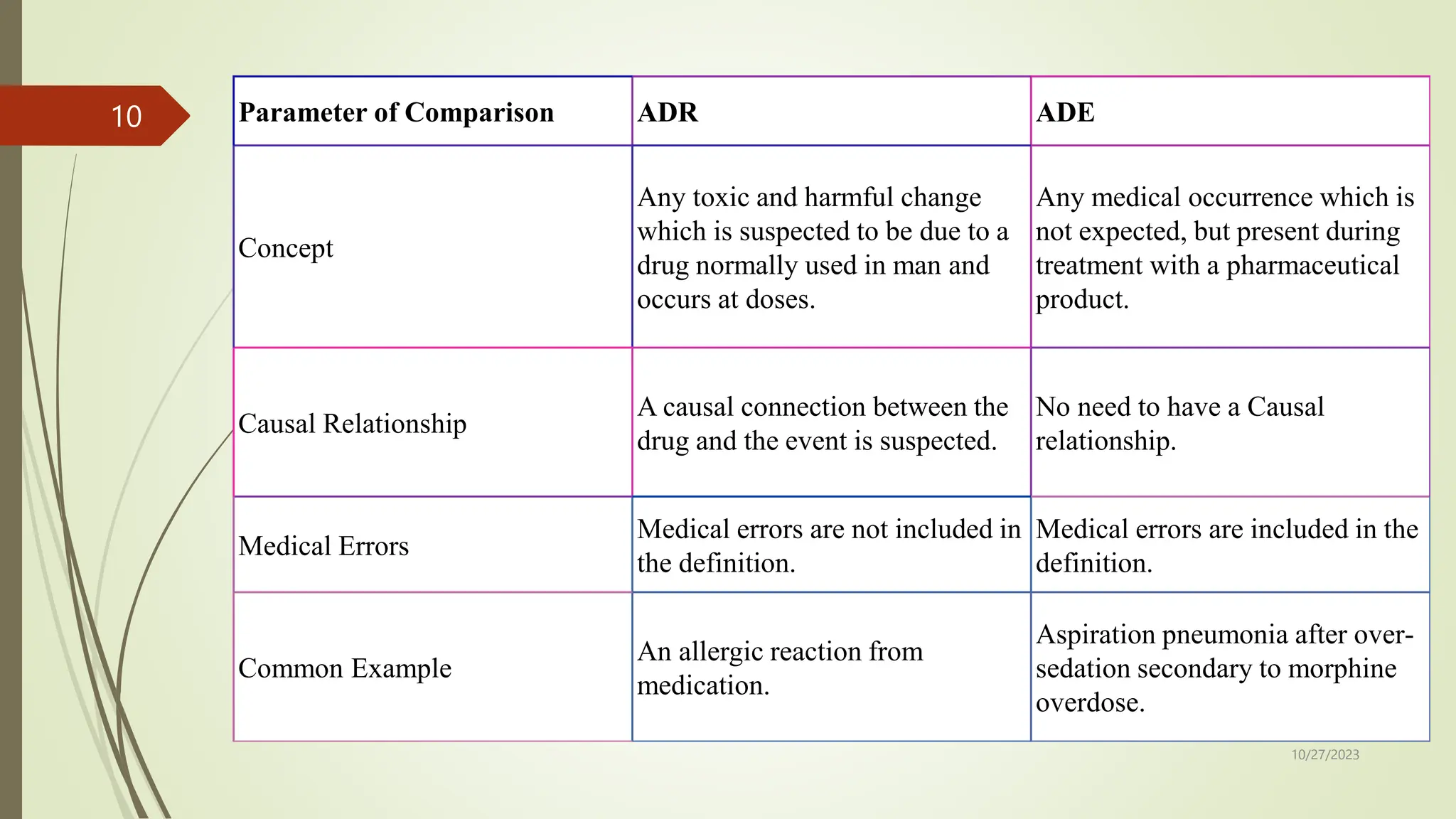
The document defines adverse drug reactions (ADRs) as any unwanted or harmful effects from a drug taken at normal doses for treatment or diagnosis of disease. ADRs can be caused by the dose, allergic reactions, or idiosyncratic reactions in individual patients. There are two major types - augmented reactions which are common and based on pharmacological properties, and bizarre reactions which are less common and require drug withdrawal. Diagnosis involves considering rechallenge or reporting to MedWatch. ADRs refer to causal harmful effects, while adverse drug events (ADEs) refer to any unfavorable medical occurrence during drug treatment whether or not causality is established, and can include medical errors.