This document provides an overview of research methodology and outlines various aspects of designing a research study, including:
- Defining experimental, quasi-experimental, and non-experimental research designs.
- Describing common data collection methods like surveys, case studies, and content analysis.
- Explaining key components of a research plan such as the research problem, objectives, background, methods, and literature cited.
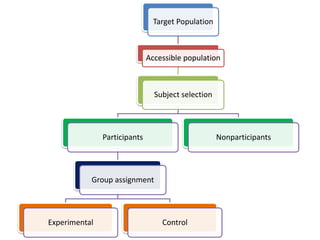
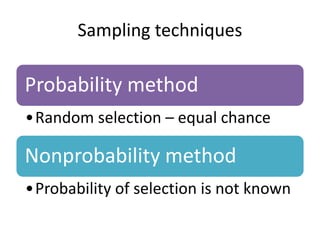
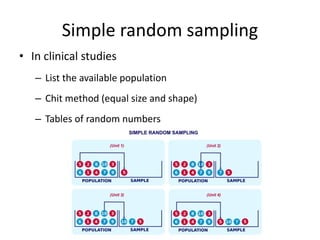
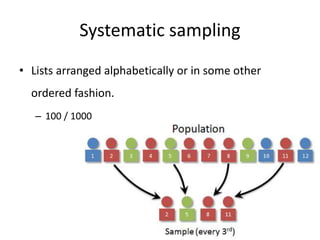
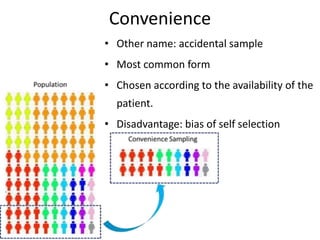
- Detailing important considerations for sampling, ensuring the normality of data distribution, and selecting appropriate statistical tests for analysis.
- Providing guidance on effectively presenting research findings through elements like visuals, abstracts, introductions, results and discussions.