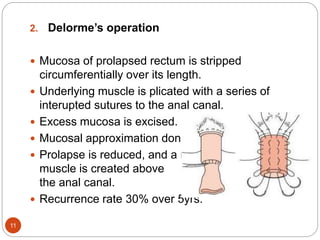
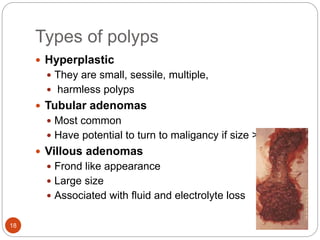
This document discusses various rectal diseases including prolapse, proctitis, polyps, benign lesions, and carcinoma. Rectal prolapse is classified as mucosal or full-thickness and can be caused by straining, weak pelvic floor muscles, or trauma from childbirth. Treatment depends on the type but may include injections, banding, or surgery. Proctitis is inflammation that can be caused by infection, radiation, or inflammatory bowel disease. Polyps are growths that can be removed endoscopically if small or via surgery if large. Benign lesions include endometriosis, hemangiomas, and neuroendocrine tumors. Rectal carcinoma is often treated with surgery such as anterior resection or