This document provides guidelines on product recalls, including:
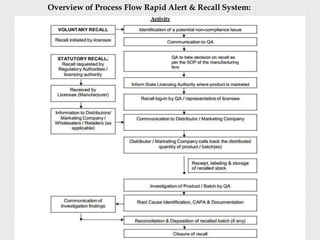
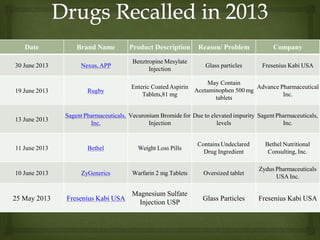
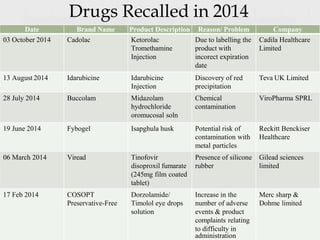
- Defining a product recall as withdrawing or removing drugs from distribution or use due to quality, efficacy, or safety issues.
- Describing the different types and levels of recalls, from compulsory recalls ordered by authorities to voluntary recalls initiated by manufacturers.
- Outlining the classification of recalls from Class I recalls for products that could cause serious health issues to Class III for minor issues.
- Providing steps for conducting an effective recall, including developing a recall strategy, recovering and storing products, notifying consumers, and evaluating the recall's effectiveness.