Embed presentation



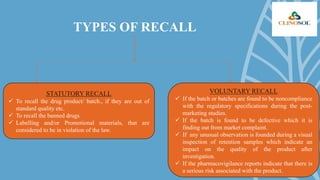
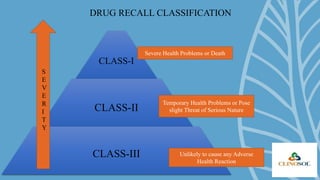
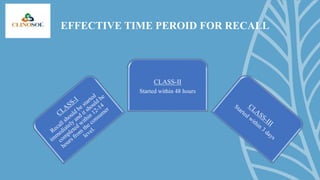
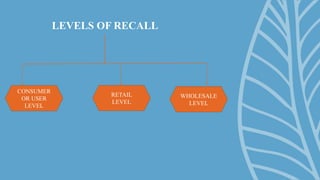



A drug recall is the removal of hazardous drugs from distribution due to issues related to quality, efficacy, or safety, including adverse reactions and defects. Recalls can be voluntary or statutory and are classified into three classes based on severity, with specific procedures for execution. Key reasons for recalls include regulatory noncompliance, consumer complaints, and ongoing testing results.









