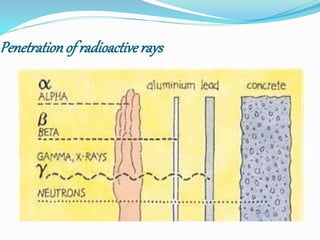
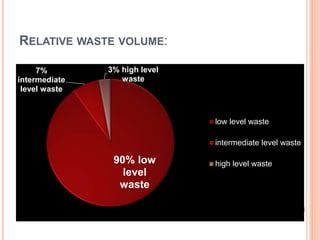
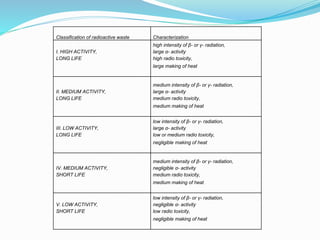
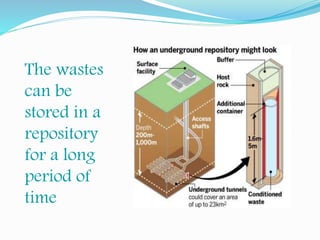
The document discusses radioactive waste, its types, and the associated risks to human health and the environment. It highlights the need for proper disposal methods, most notably geological disposal, and introduces bioremediation techniques using microorganisms to reduce hazardous materials in nuclear waste. Specific bacteria are mentioned that can decompose harmful substances like mercury and cyanide, and the potential for bacteria to precipitate radionuclides like uranium is also explored.