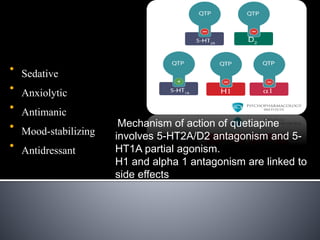
Quetiapine is a first-line atypical antipsychotic that has been used since 1998 for schizophrenia, bipolar disorder, depression, and other conditions. It is absorbed quickly in the body and metabolized in the liver. Quetiapine acts as an antagonist at serotonin, histamine, alpha-1, and dopamine receptors, with varying degrees of affinity. Common side effects include sedation, dizziness, hypotension, weight gain, and metabolic changes. Overdoses may cause lethargy, tachycardia, respiratory issues, and other symptoms, but are typically not fatal.