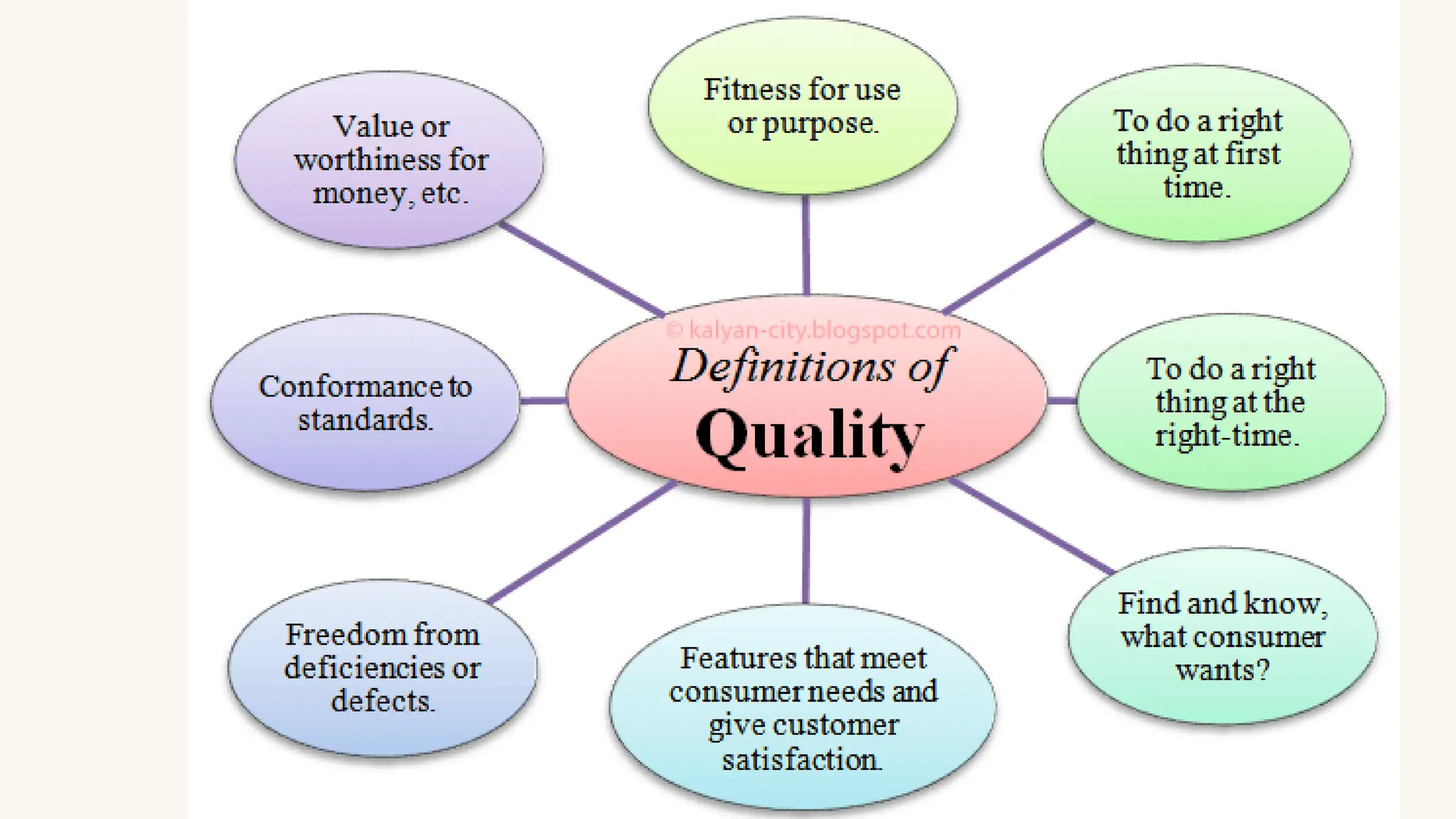
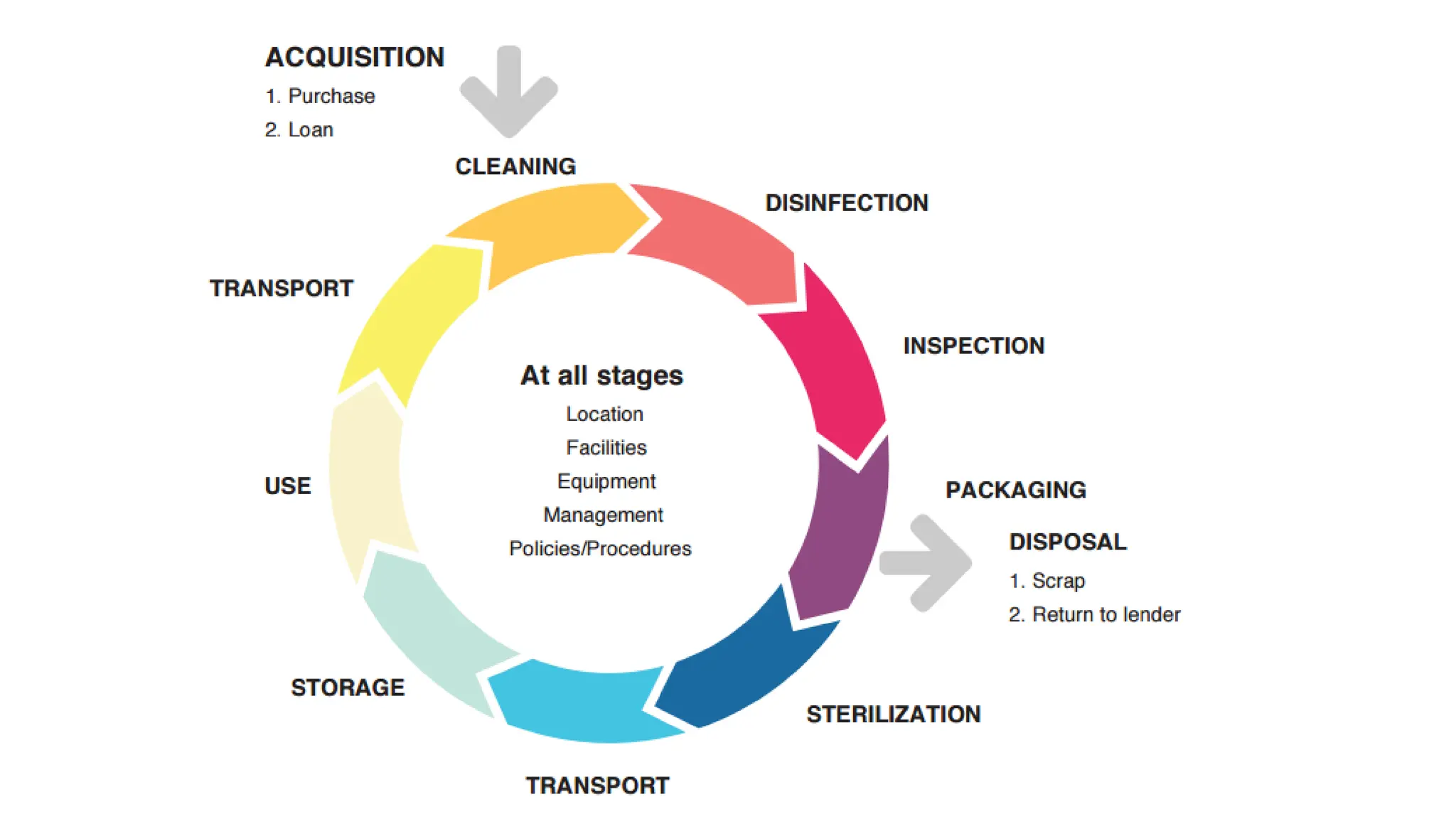
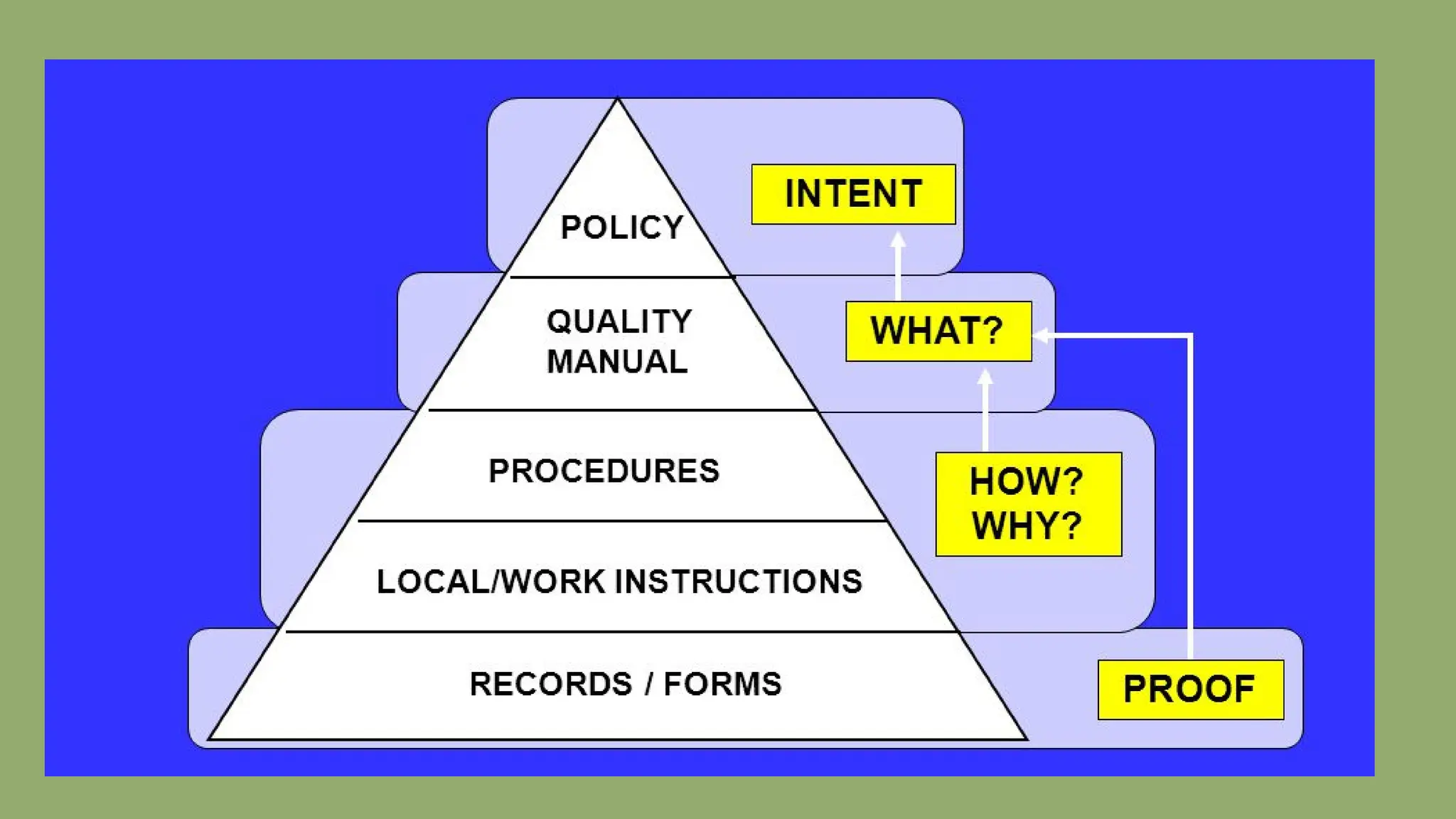
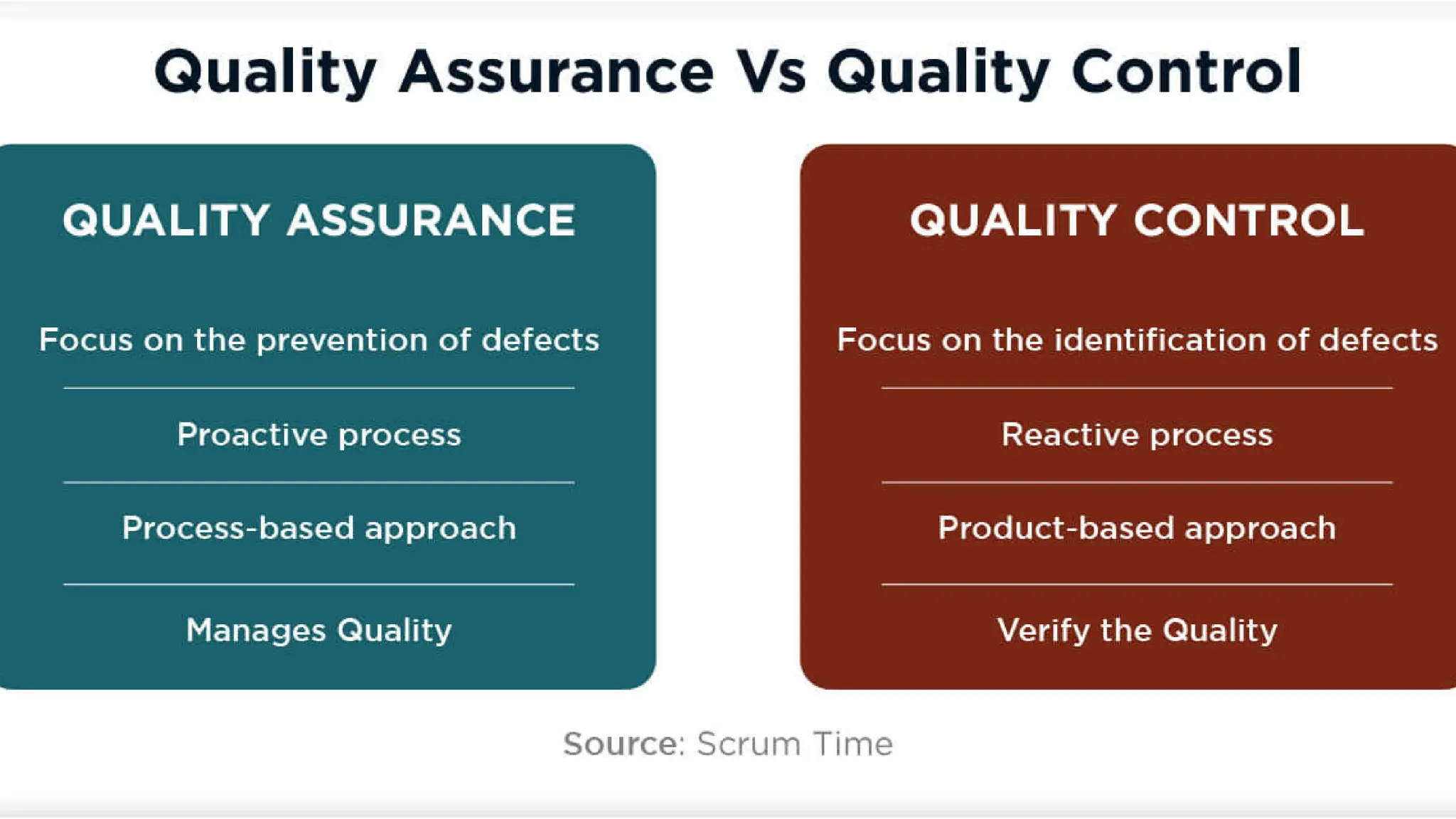
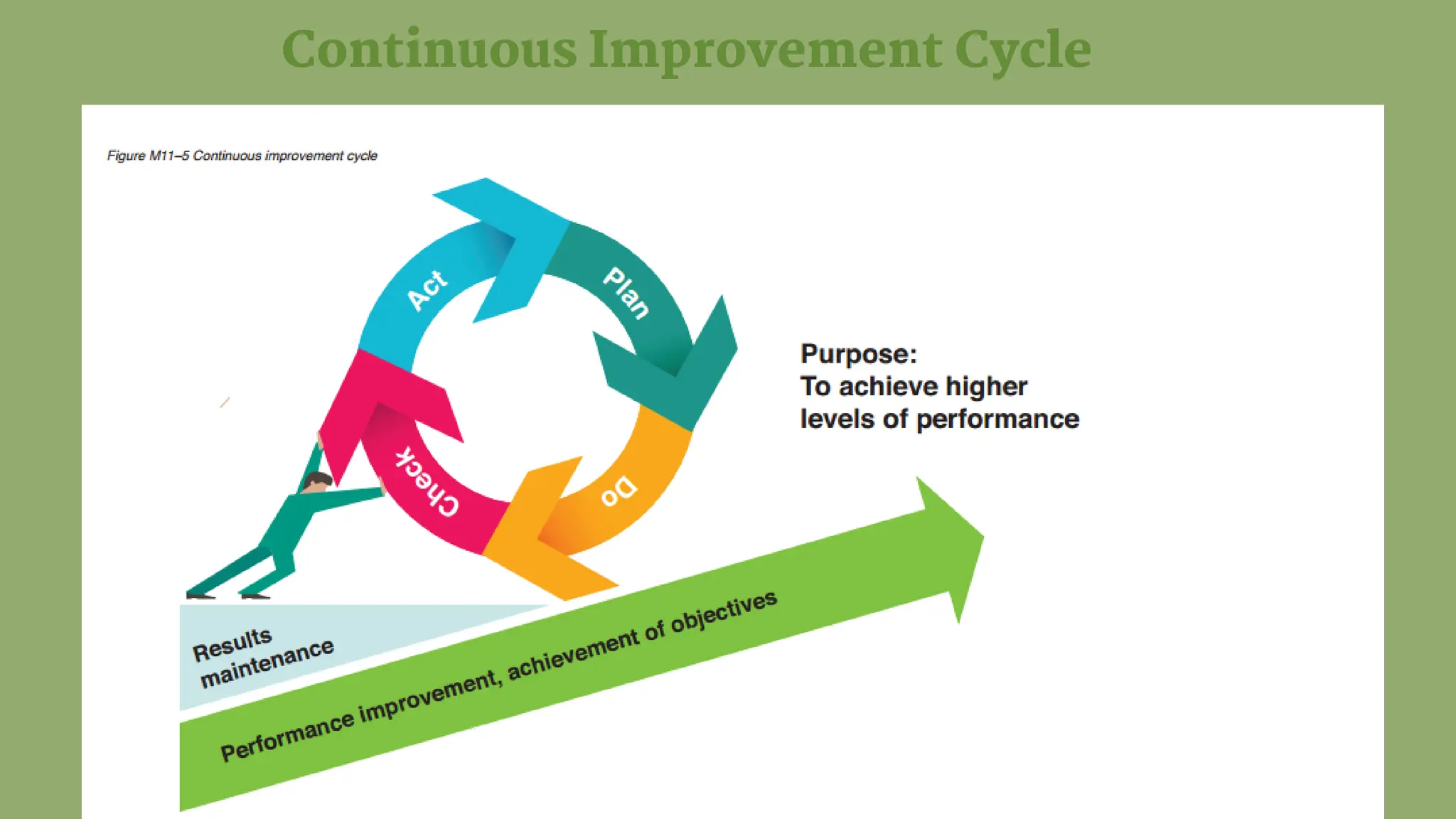
The document details the quality assurance goals and objectives for central sterile supply departments (CSSDs), emphasizing the importance of quality management systems, documentation, and staff training to ensure reusable medical devices are 'fit for purpose.' It distinguishes between quality assurance, which focuses on preventing defects, and quality control, which identifies them, while also highlighting the need for continuous performance monitoring and improvement. Key processes such as defect reporting, product recall systems, and electronic tracking are outlined to maintain high standards in device sterilization and customer service.