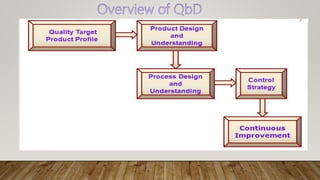
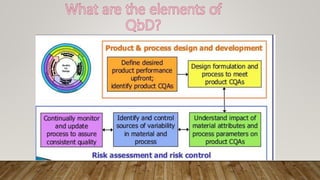
The document discusses Quality by Design (QbD), which is a systematic approach to pharmaceutical development that begins with predefined objectives and emphasizes product and process understanding based on sound science and quality risk management. QbD involves determining critical quality attributes, understanding how raw materials and process parameters affect those attributes, developing a design space and control strategy to manage the product lifecycle. Design of experiments is a key QbD tool that involves screening factors, response surface methodology, formulation evaluation, computer modeling and validation to optimize the process and enable scale up.