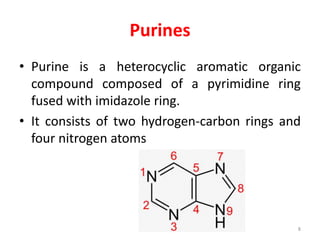
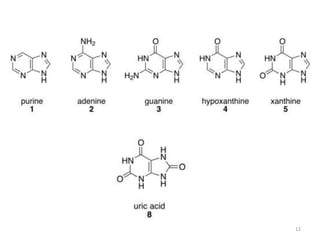
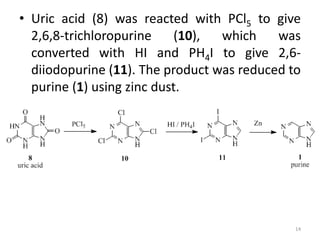
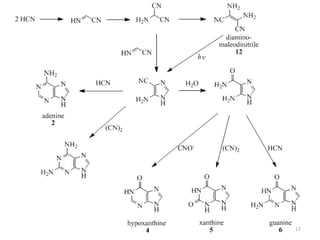
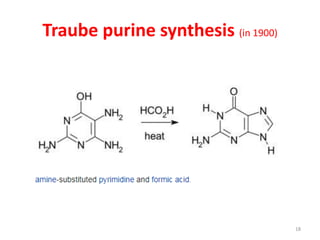
This document discusses purines, which are organic compounds that are one of the two types of nucleobases found in nucleic acids. Purines consist of fused pyrimidine and imidazole rings and contain four nitrogen atoms. Adenine and guanine are the two purine nucleobases found in DNA and RNA. The document provides details on the structure and properties of purines, their sources in food, their roles in nucleic acids, and the early history and synthesis of purine starting from uric acid.