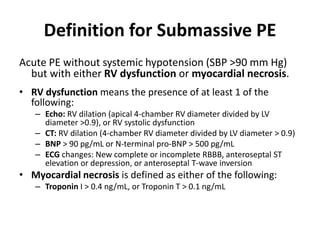
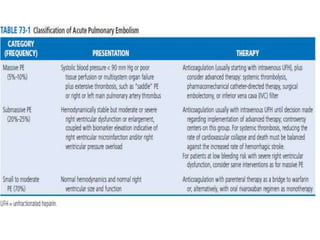
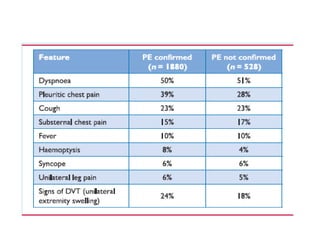
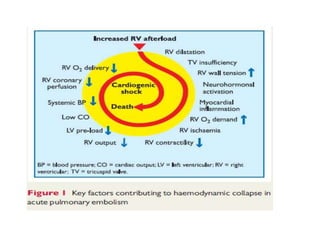
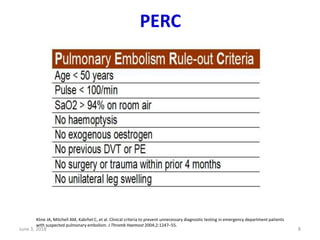
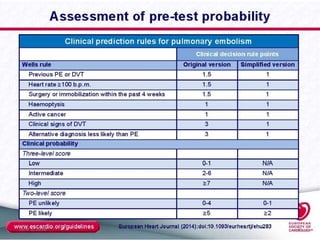
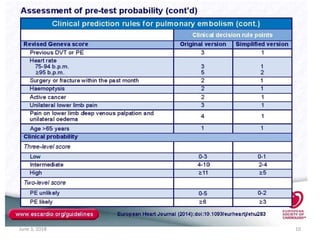
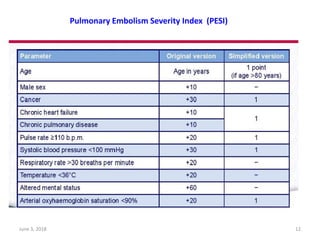
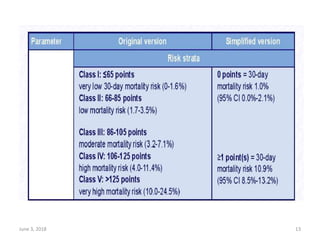
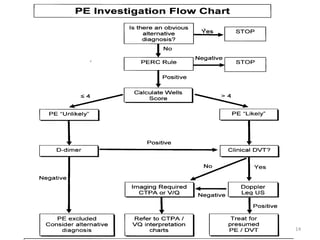
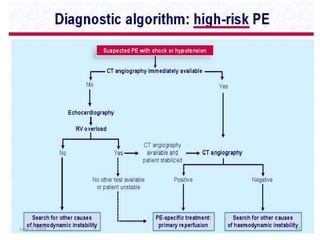
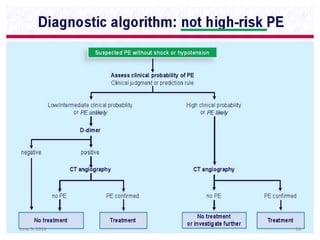
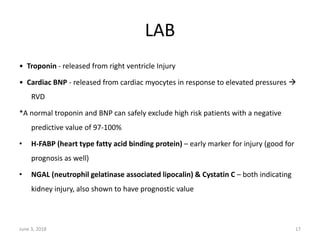
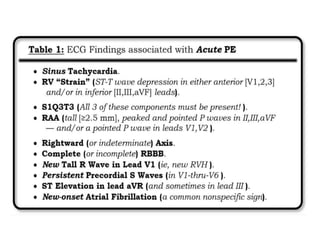
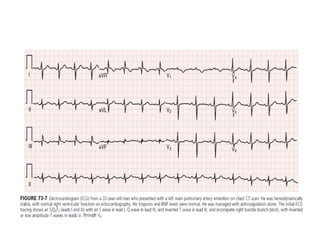
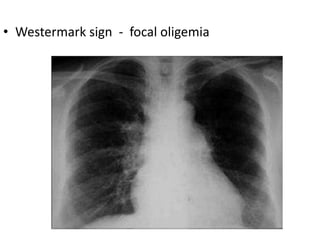
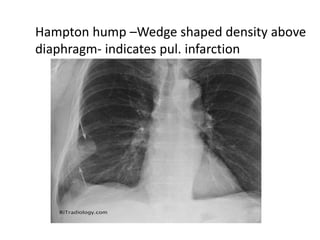
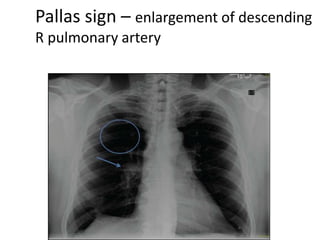
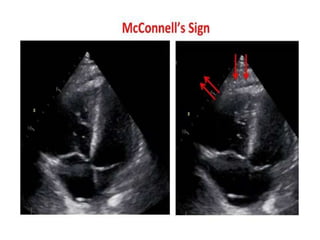
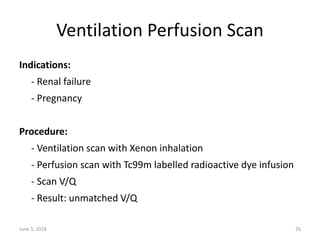
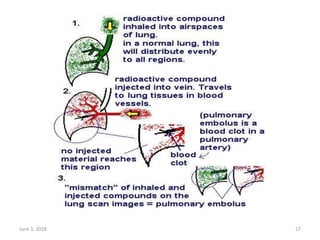
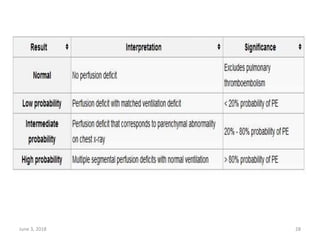
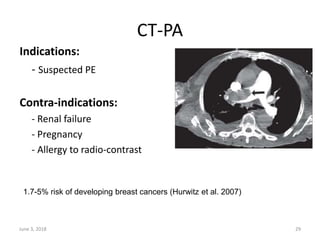
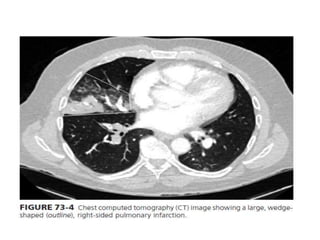
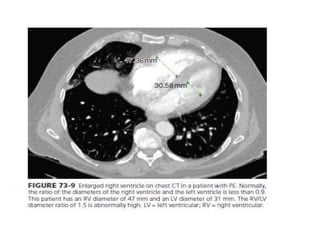
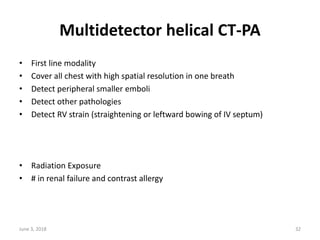
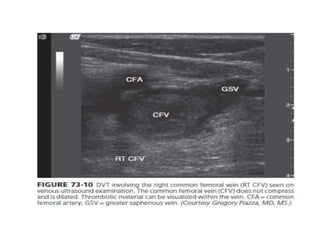
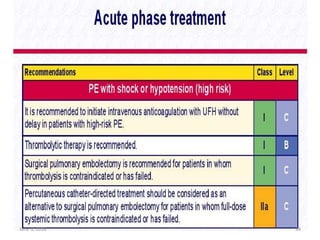
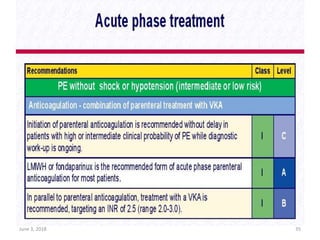
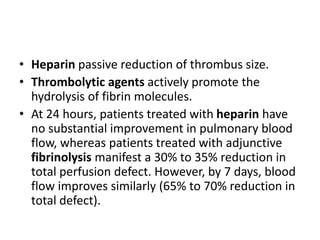
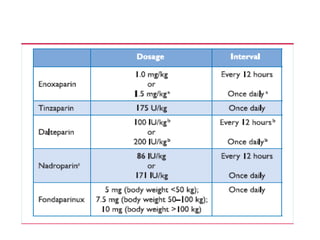
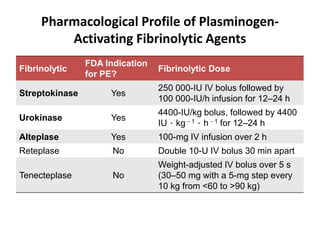
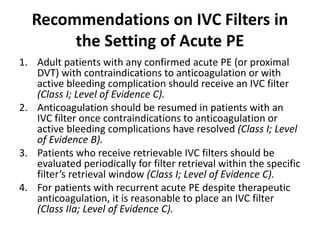
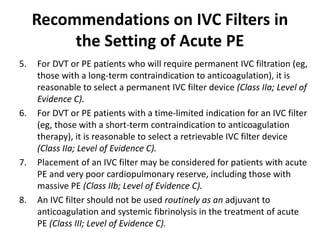
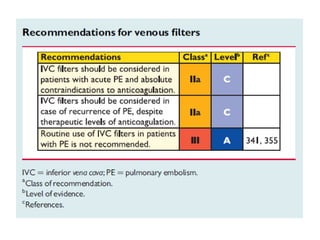
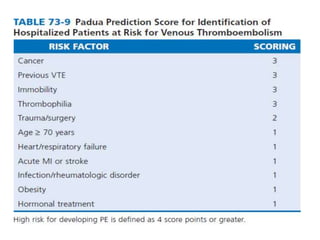
This document defines massive, submassive, and low-risk pulmonary embolism (PE) and provides diagnostic criteria for each. Massive PE is defined by sustained hypotension, pulselessness, or profound bradycardia. Submassive PE is defined by right ventricular dysfunction or myocardial necrosis without hypotension. Low-risk PE lacks the criteria for massive or submassive PE. Diagnostic tests and initial treatment recommendations are also summarized.