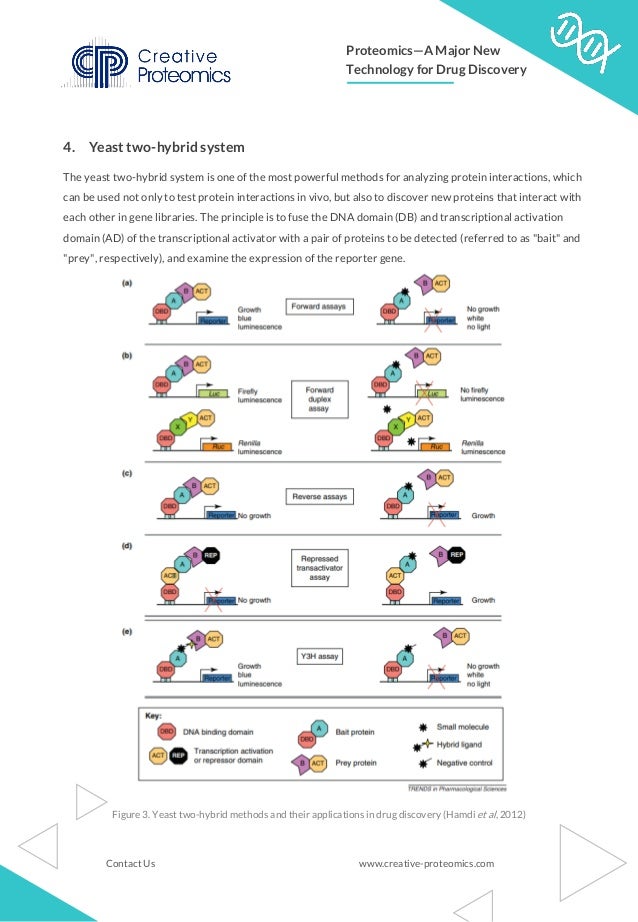
The document discusses the significance of proteomics and pharmacoproteomics in drug discovery, detailing how it analyzes protein dynamics, identifies drug targets, and optimizes lead compounds. Key techniques highlighted include two-dimensional gel electrophoresis, mass spectrometry, protein microarrays, and the yeast two-hybrid system for studying protein interactions and drug actions. Comprehensive proteomics services are provided to aid research in drug discovery processes.