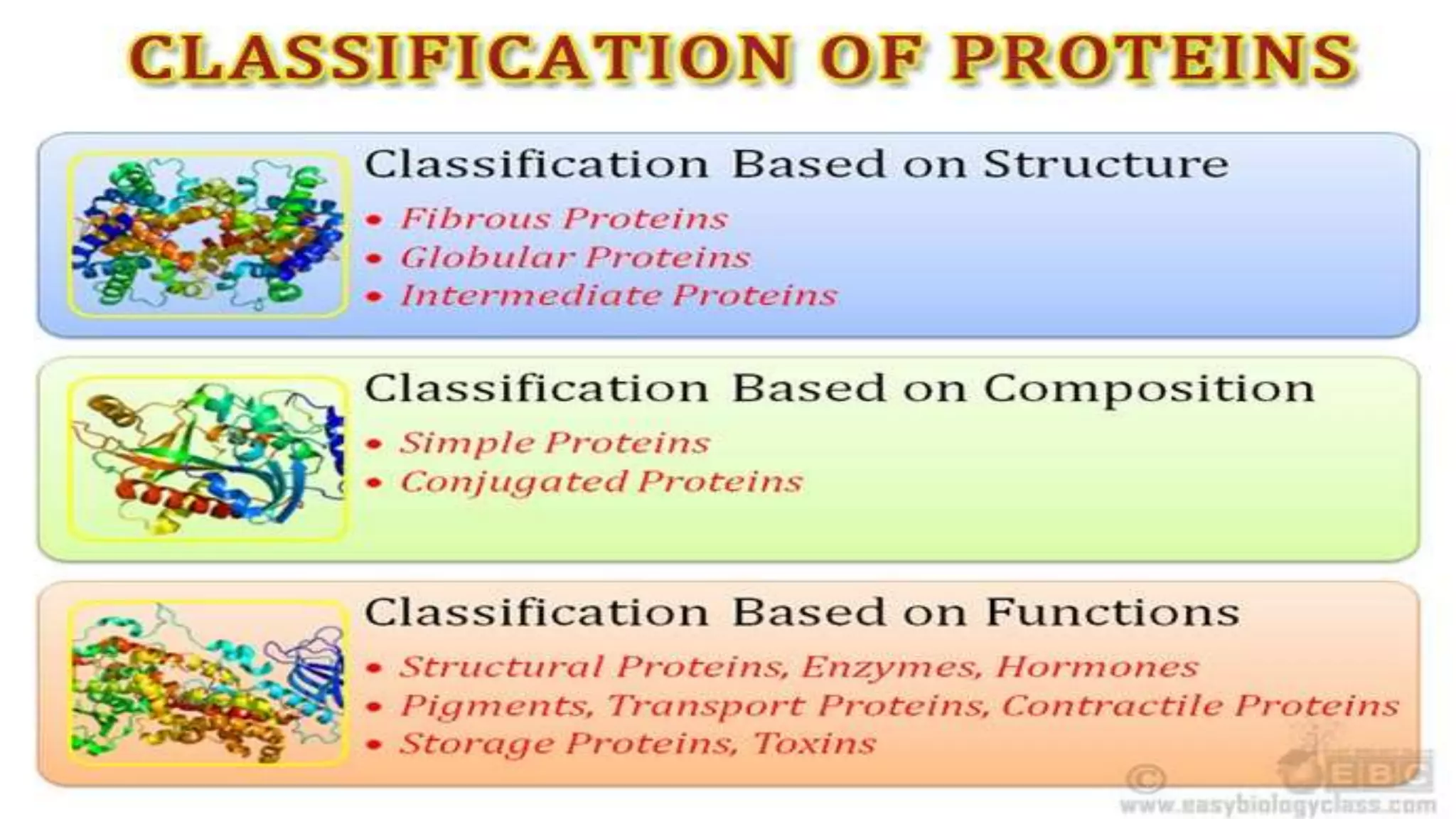
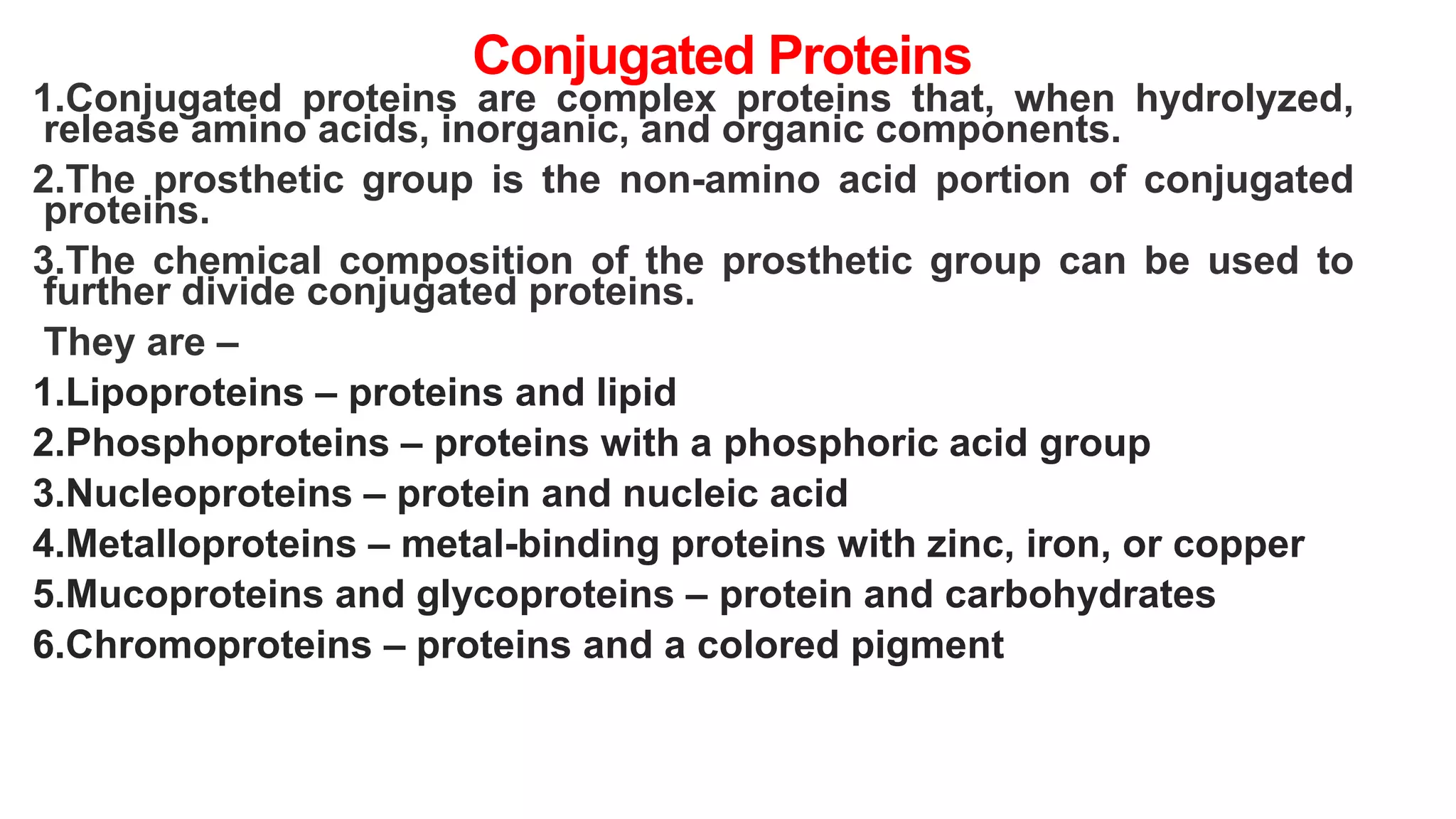
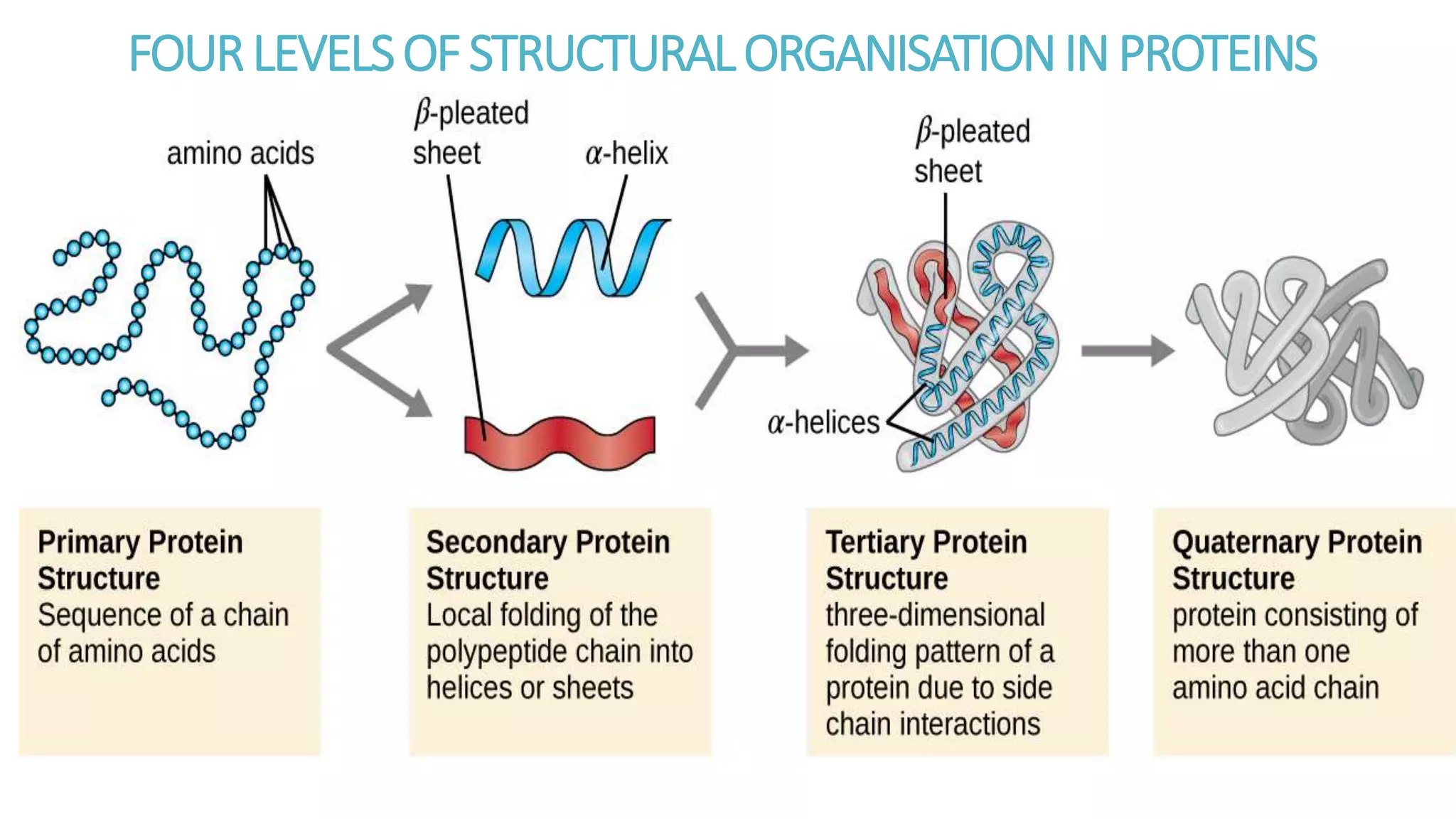
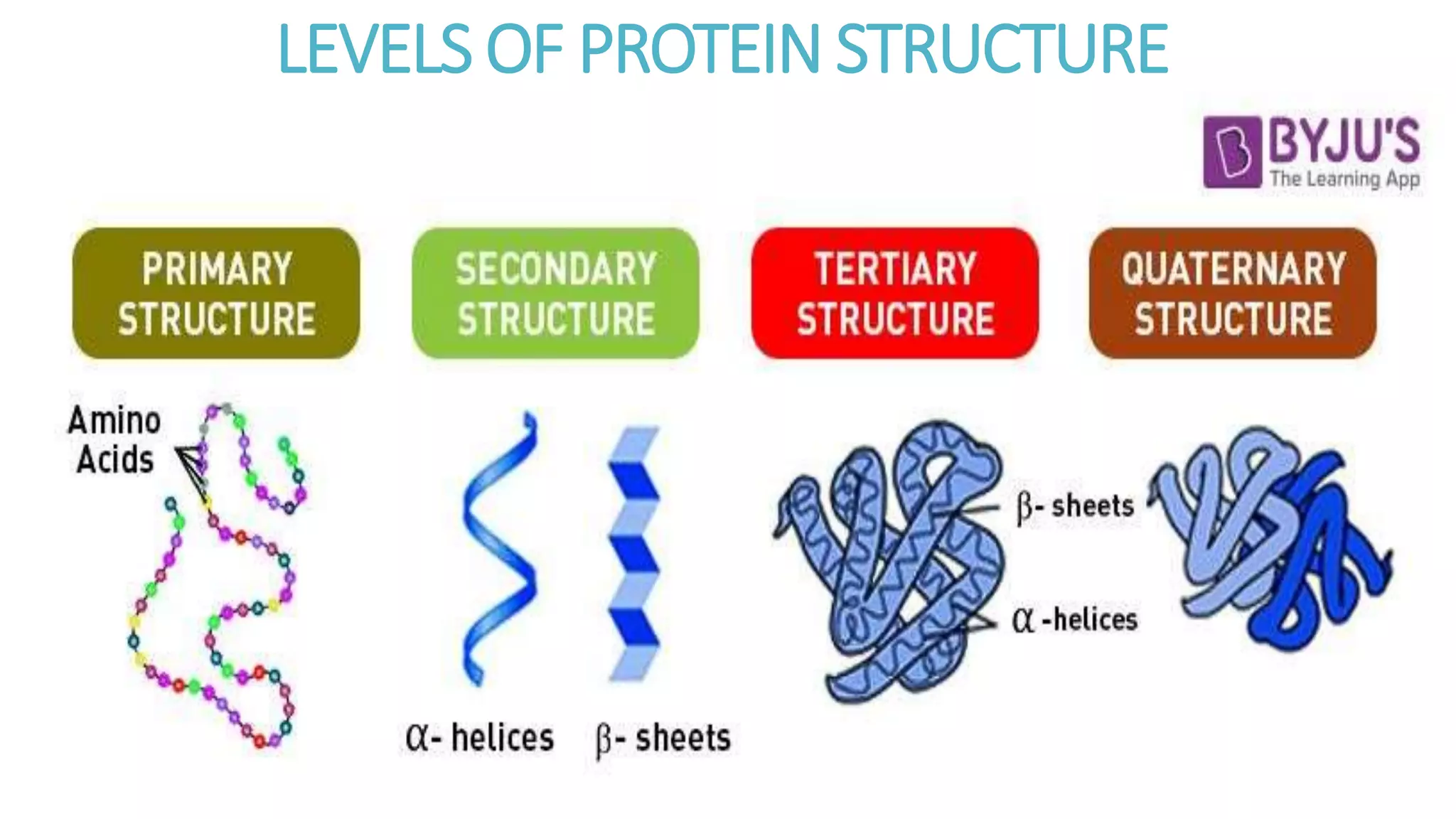
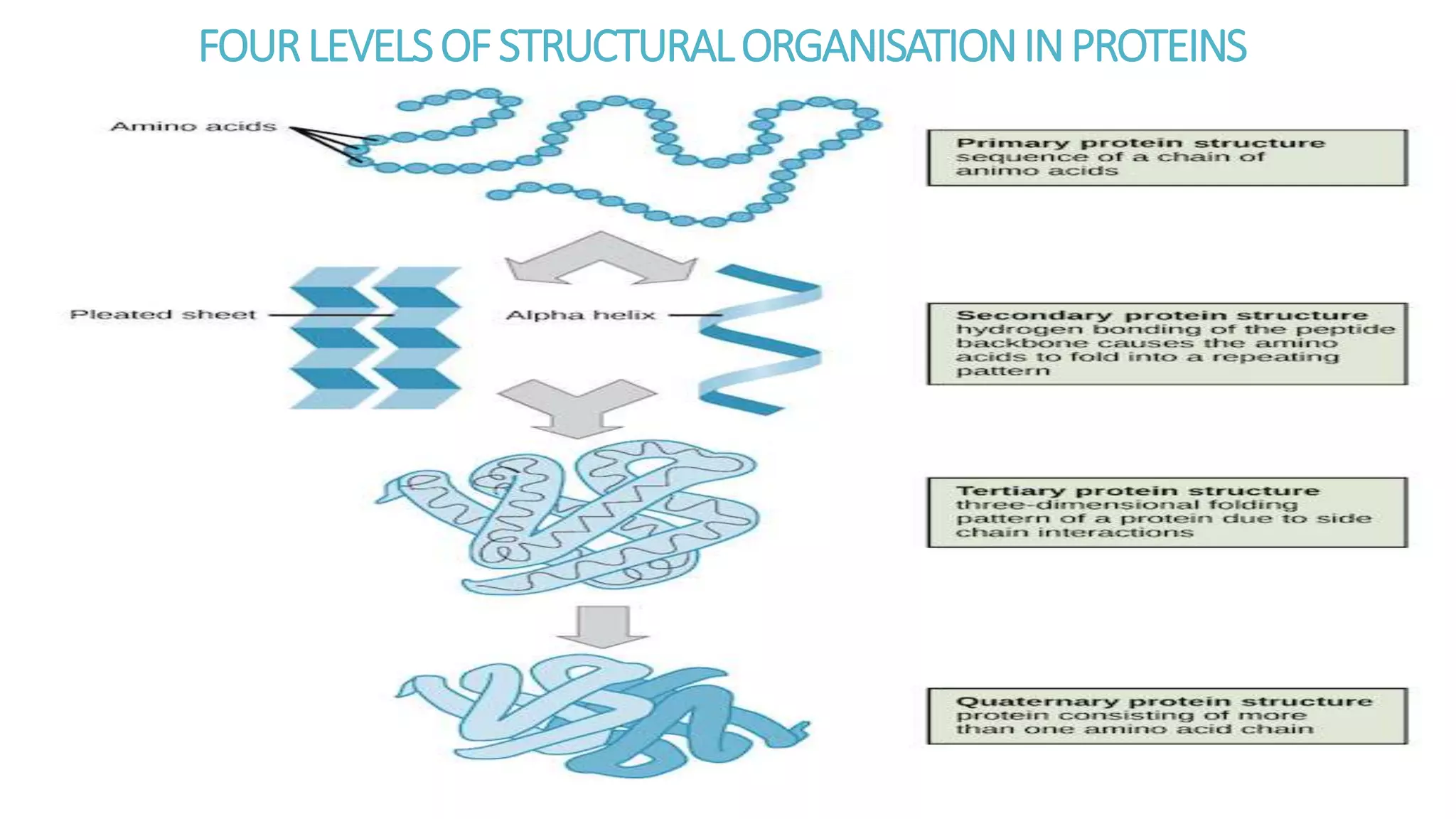
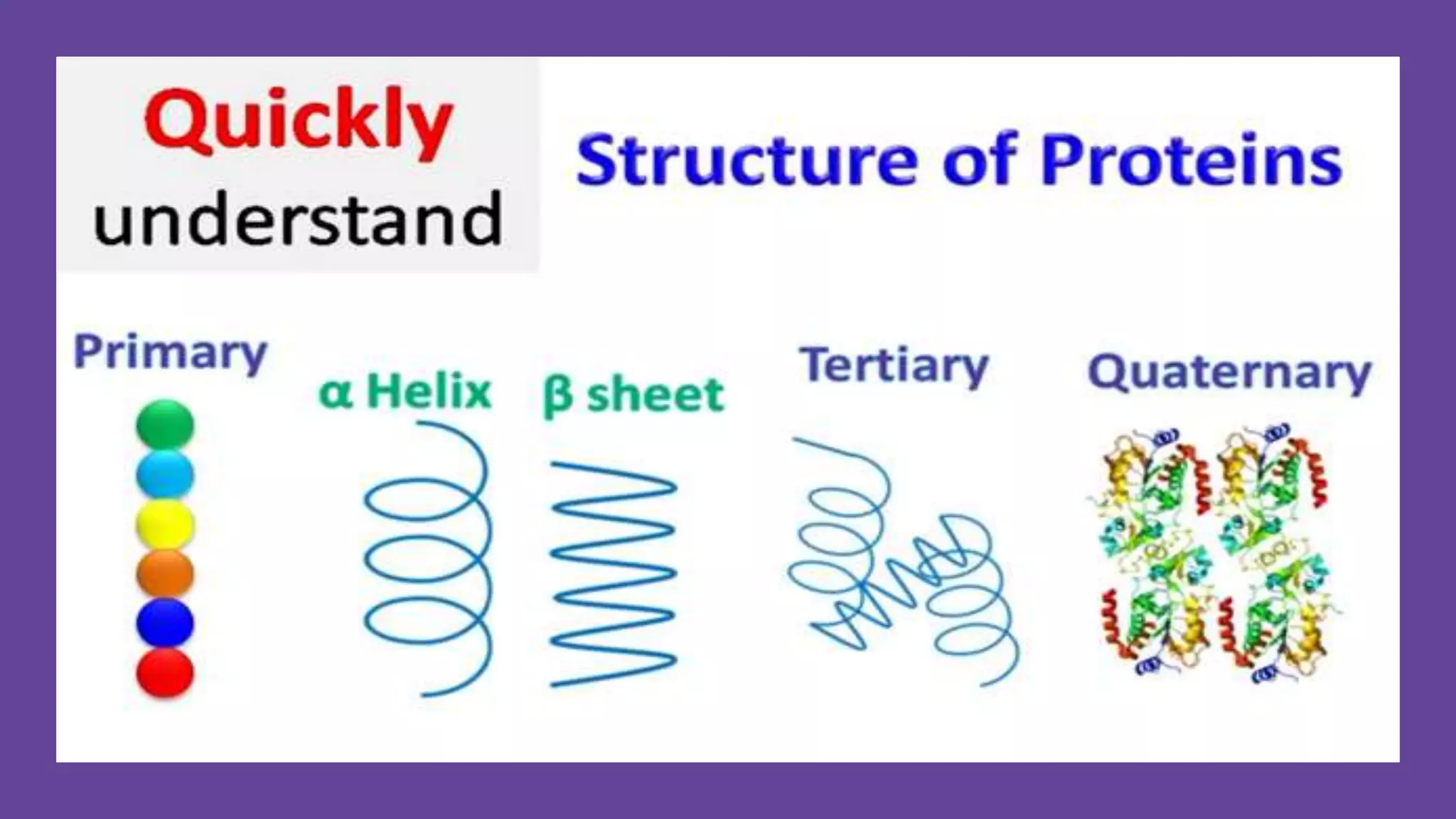
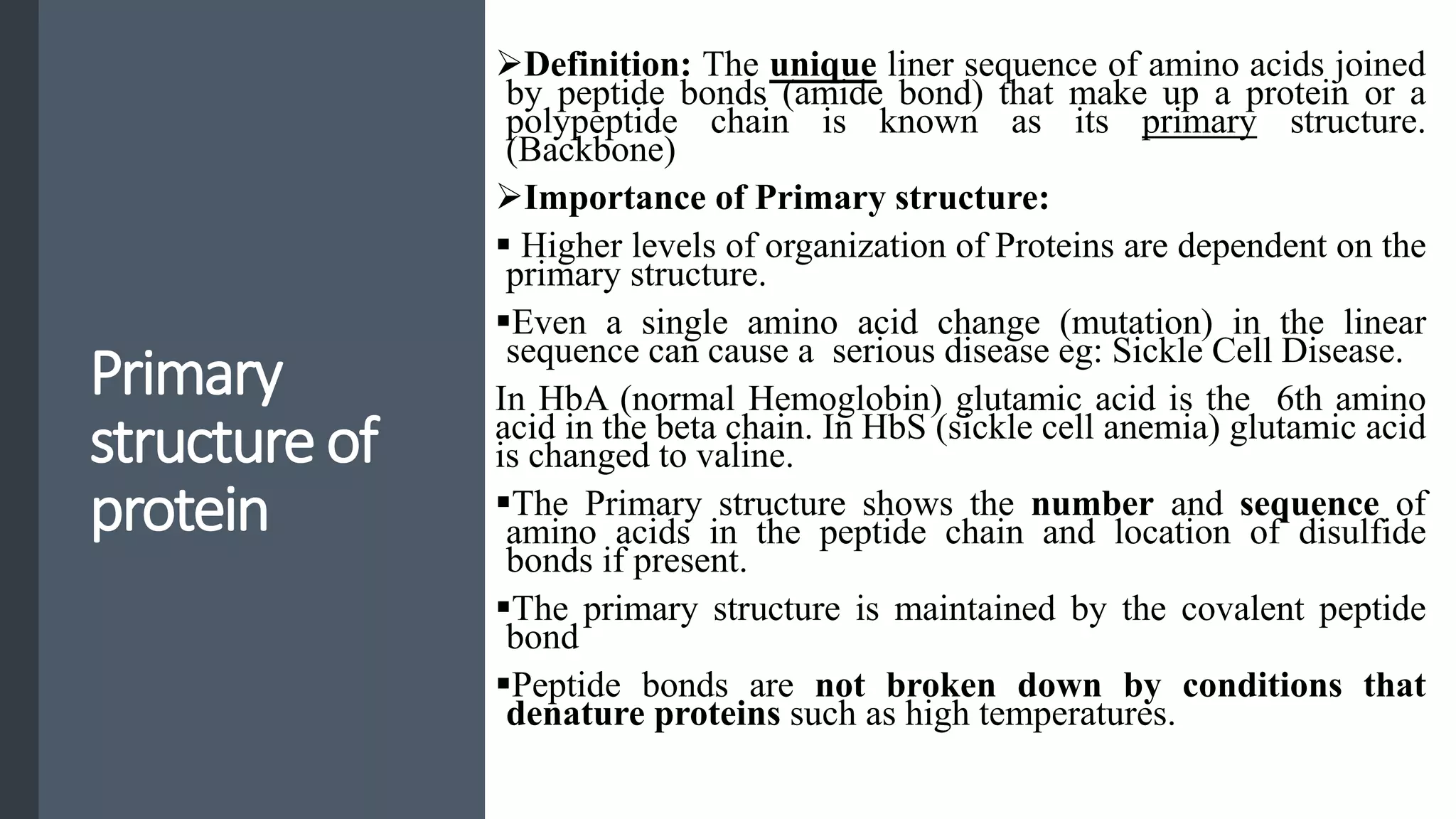
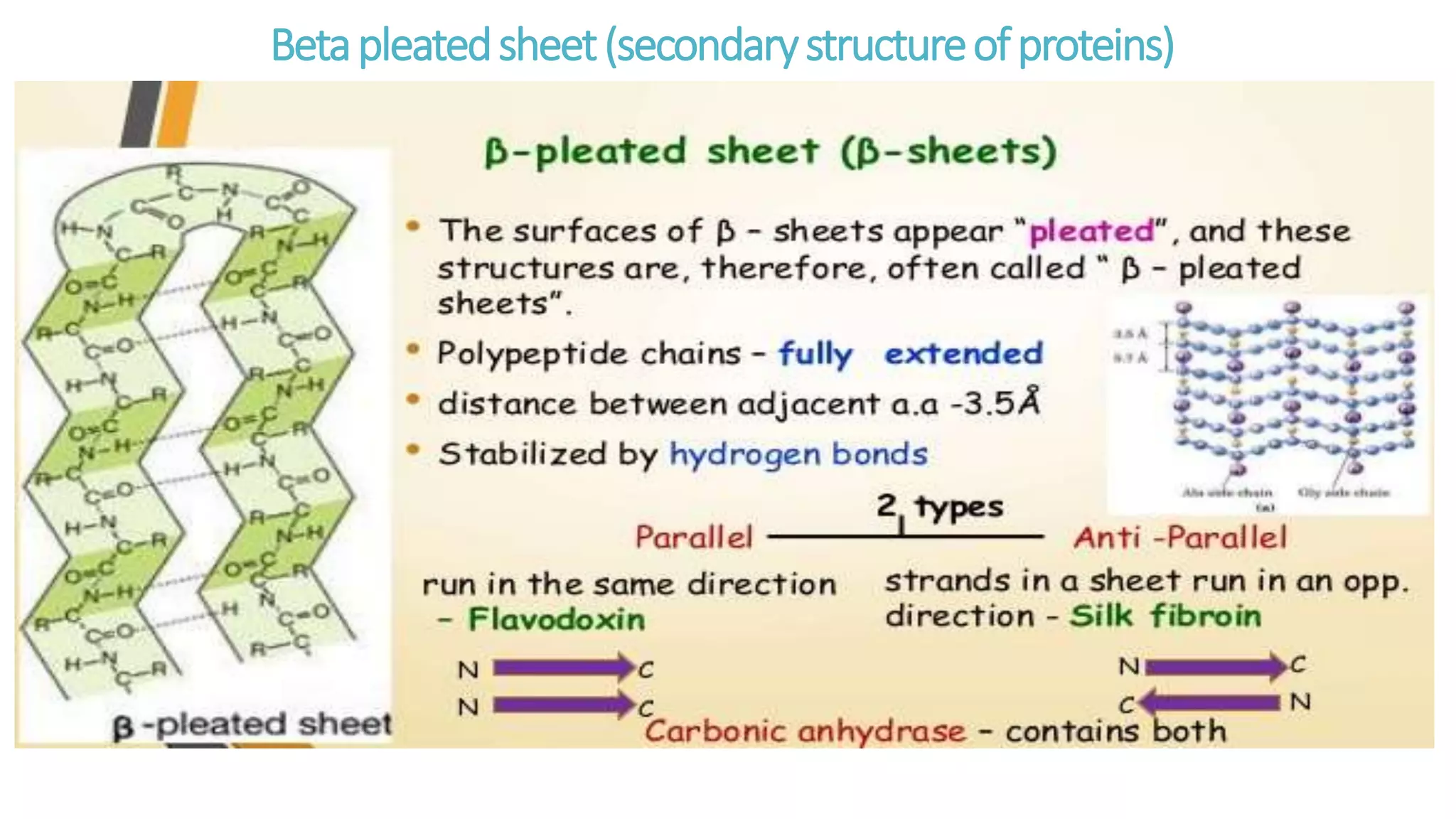
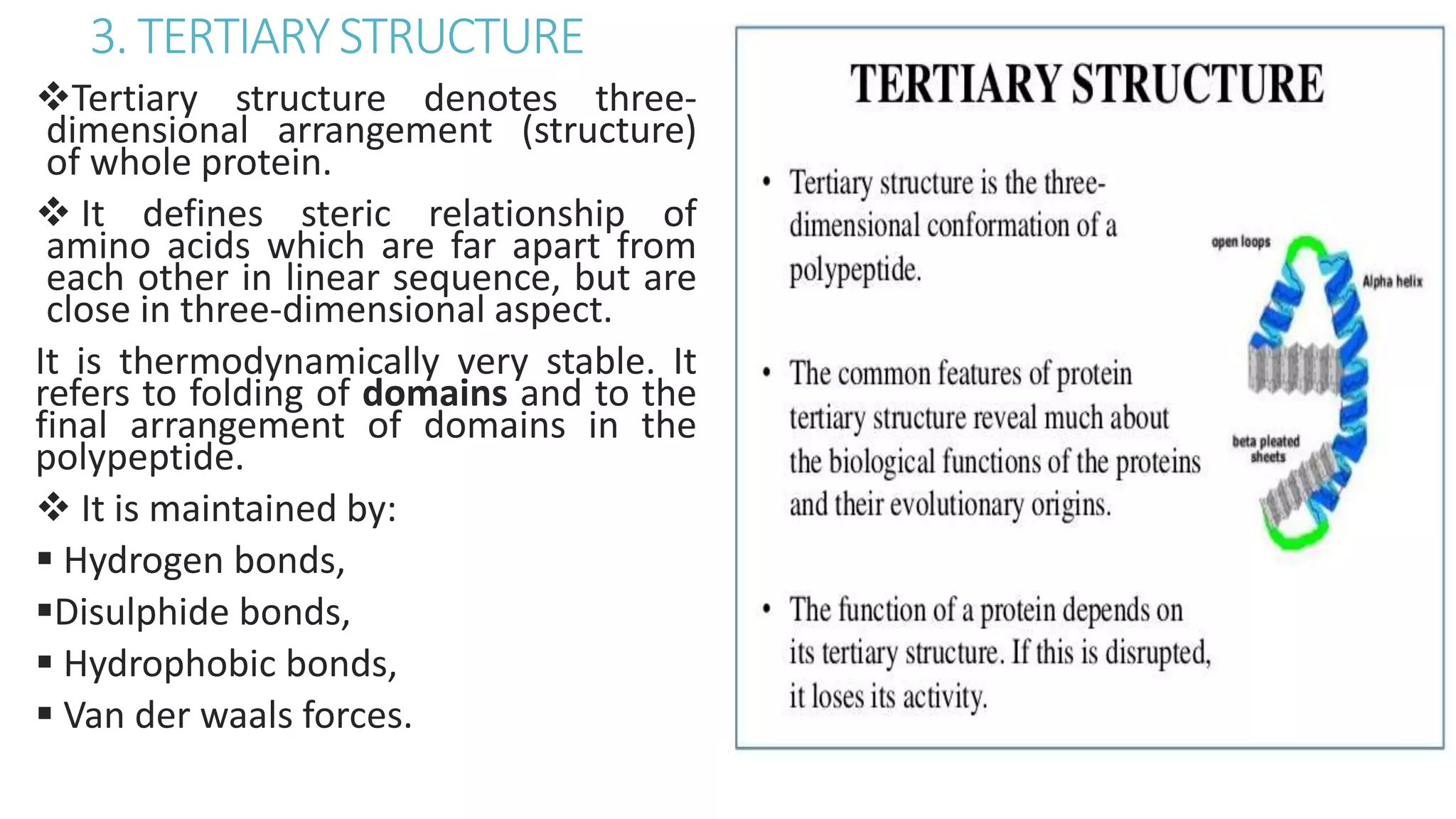
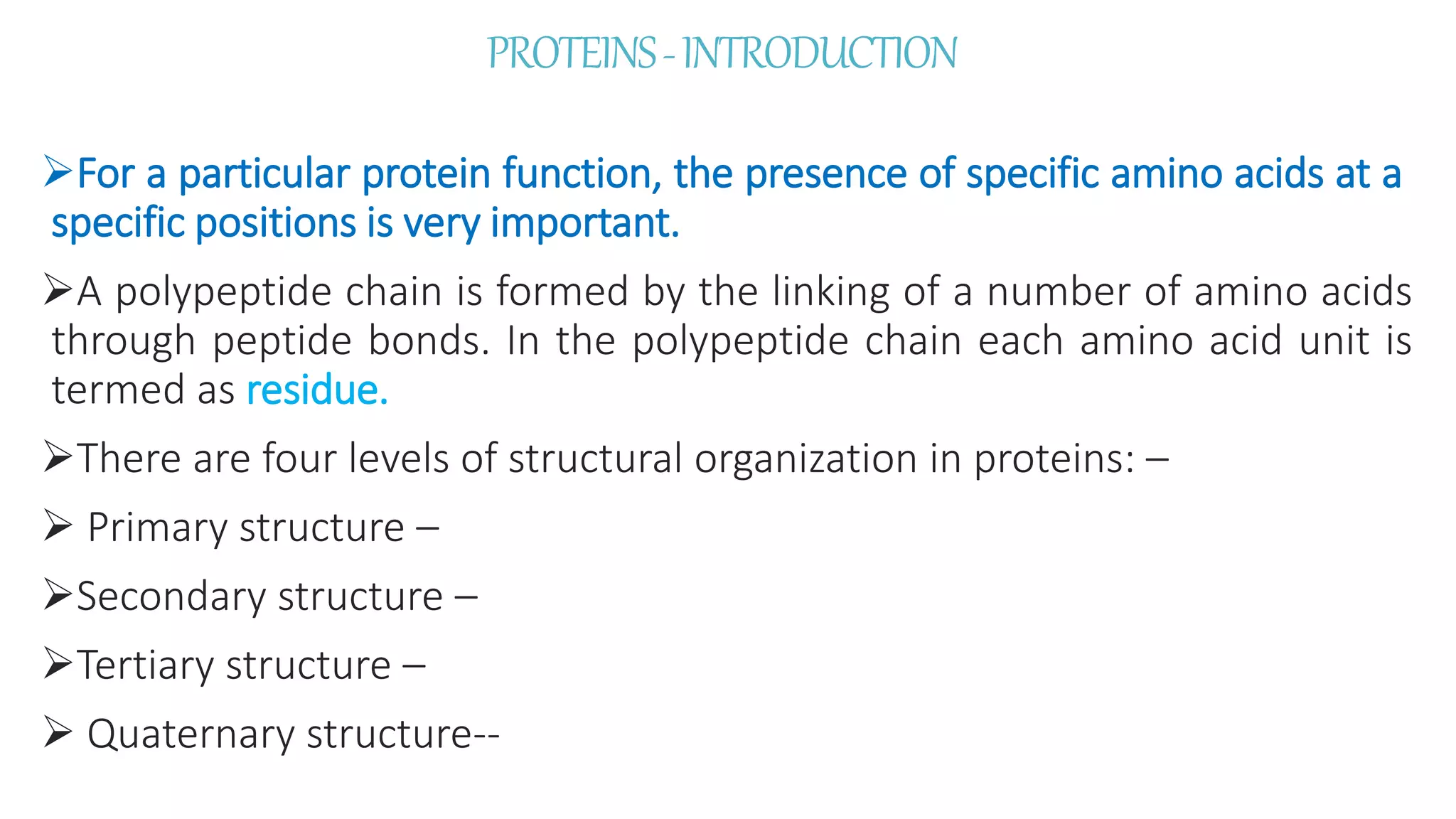
This document discusses various aspects of protein structure and function. It begins by defining proteins and describing their essential roles in the body. It then covers the four levels of protein structural organization: primary, secondary, tertiary, and quaternary structure. Specific examples are provided to illustrate alpha helices, beta sheets, and triple helical structures. The document also discusses protein classification, functions, and conjugated proteins. Overall, it provides a comprehensive overview of the key concepts regarding protein structure and the important roles of proteins in biological systems.







![AMIDE(PEPTIDE)BONDFORMATION
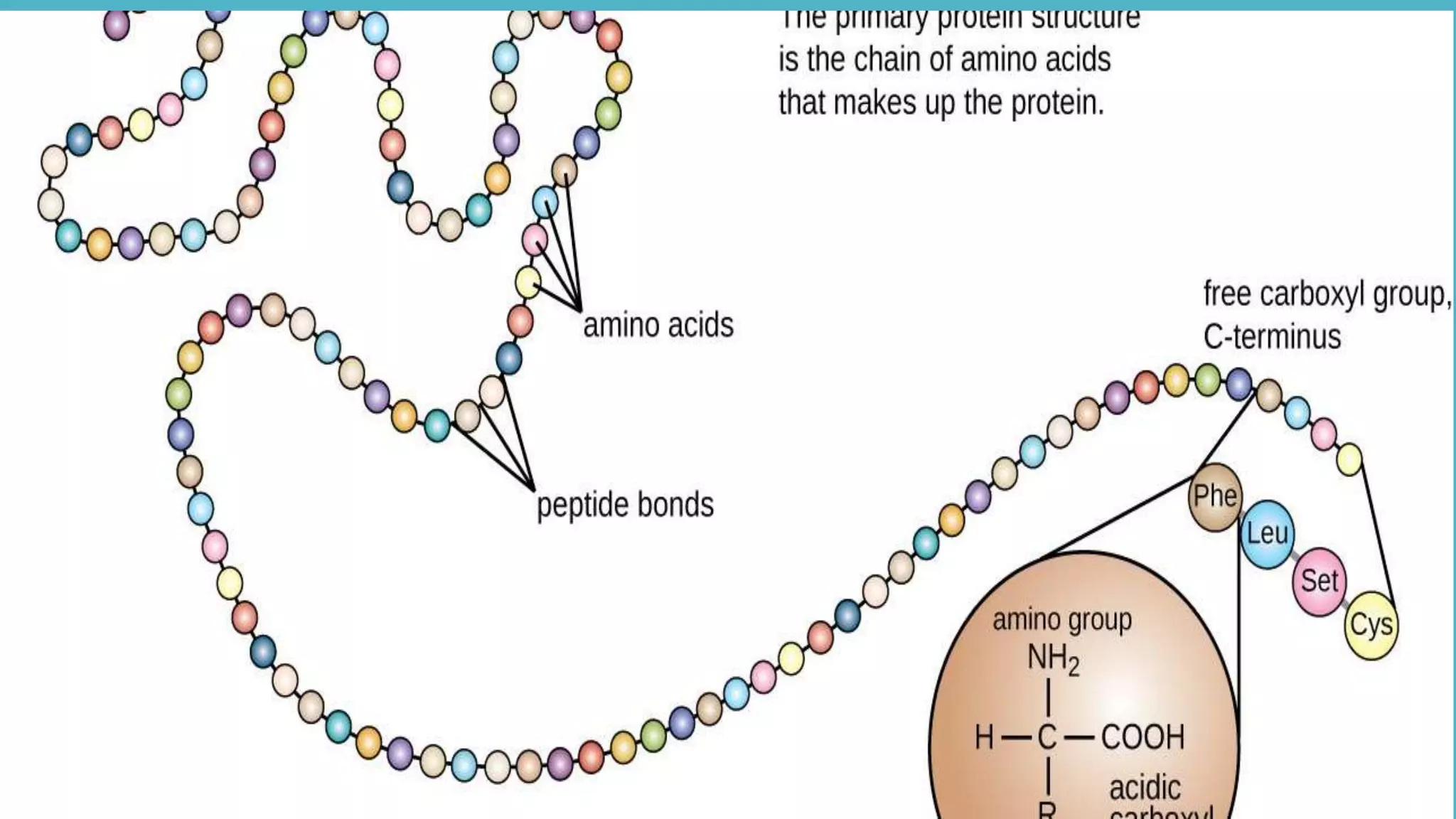
The primary sequence of a protein is formed by the linkage of
the carboxylic acid group of Amino acid 1 with the amine
functional group of the Amino acid 2 to form an amide
(Peptide)linkage [through a dehydration synthesis (loss of
water)] .
Similarly, the reverse reaction is hydrolysis and requires the
incorporation of a water molecule to separate two amino acids
and break the amide bond.
Notably, peptidyl transferase (in the ribosome) serves as the
enzyme that mediates the dehydration synthesis reactions
required to build protein molecules, whereas a class of
enzymes called proteases are required for protein hydrolysis.](https://image.slidesharecdn.com/proteinslevelsofstructuralconformationpptx-230311114108-8fc256cc/75/PROTEINS-LEVELS-OF-STRUCTURAL-CONFORMATION-pptx-pptx-9-2048.jpg)