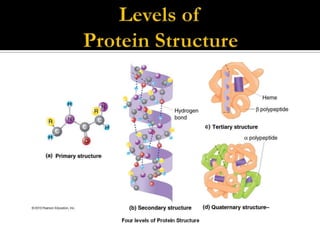
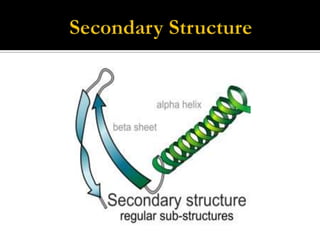
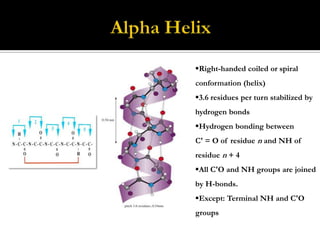
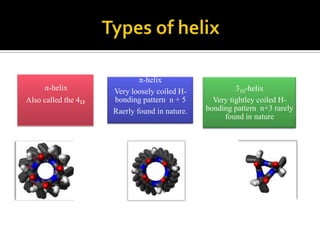
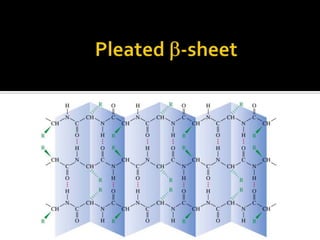
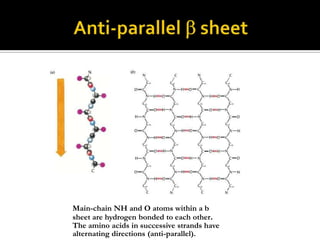
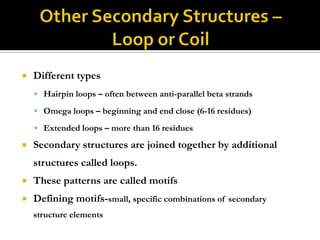
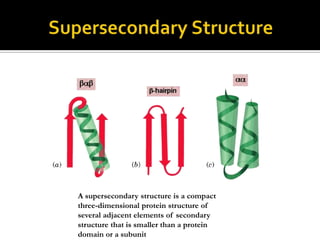
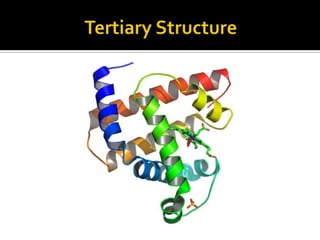
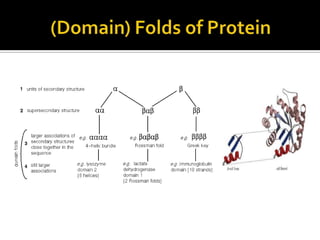
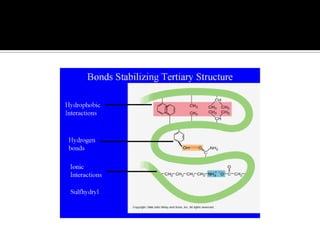
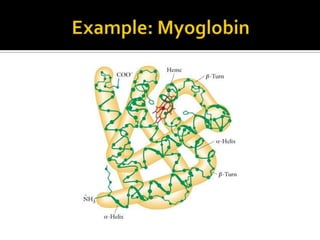
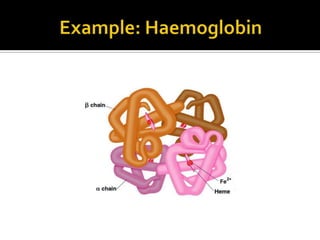
Protein structure can be described at several levels of organization. The primary structure is the amino acid sequence, while the secondary structure describes local patterns like alpha helices and beta sheets formed by hydrogen bonds. Tertiary structure refers to the overall 3D shape of a single polypeptide chain. Quaternary structure involves the arrangement of multiple protein subunits. Together these organizational levels allow proteins to carry out their diverse functions in the cell.