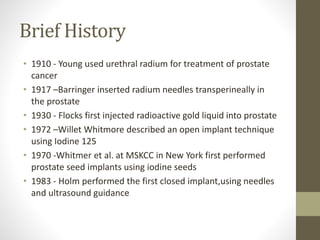
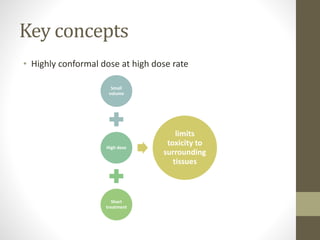
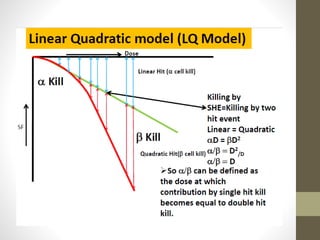
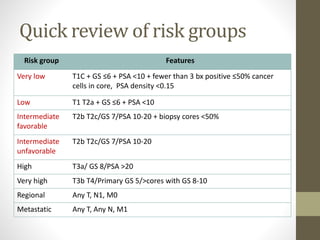
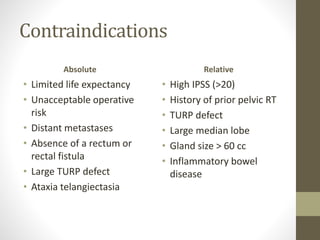
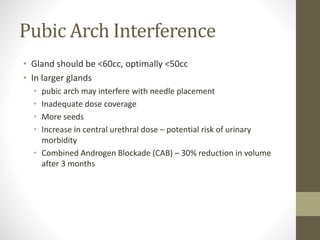
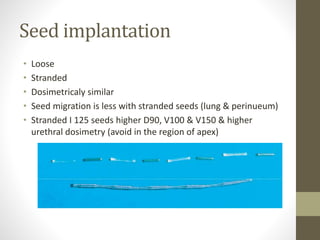
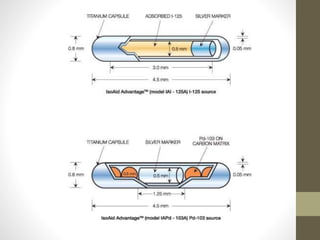
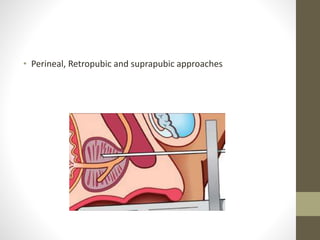
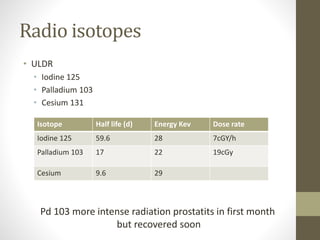
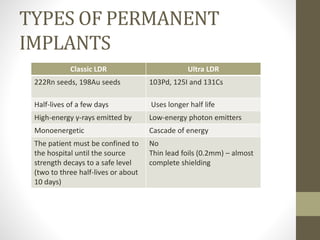
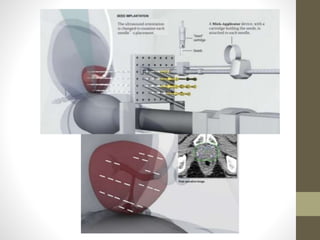
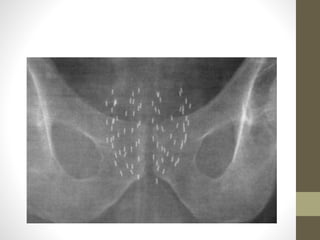
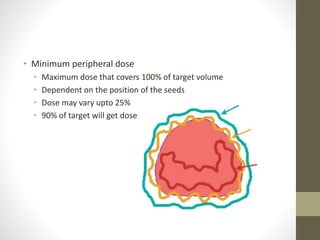
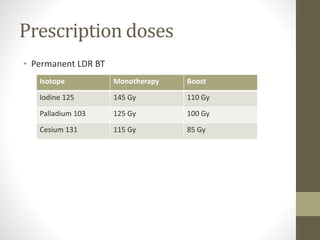
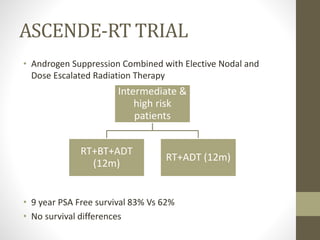
Brachytherapy is an excellent treatment for prostate cancer that provides long term tumor control comparable to radical prostatectomy and external beam radiation therapy. It involves placing radioactive sources directly in the prostate gland temporarily or permanently. Common radioactive sources include iodine-125 and palladium-103 seeds. Brachytherapy can be used as monotherapy for low risk prostate cancer or as a radiation boost combined with external beam radiation for higher risk disease. It allows a highly conformal dose to be delivered to the prostate while sparing surrounding tissues from radiation exposure. Brachytherapy is generally a low risk treatment with most side effects being temporary increased urinary symptoms. It provides patients an alternative to surgery or external beam radiation for localized prostate cancer