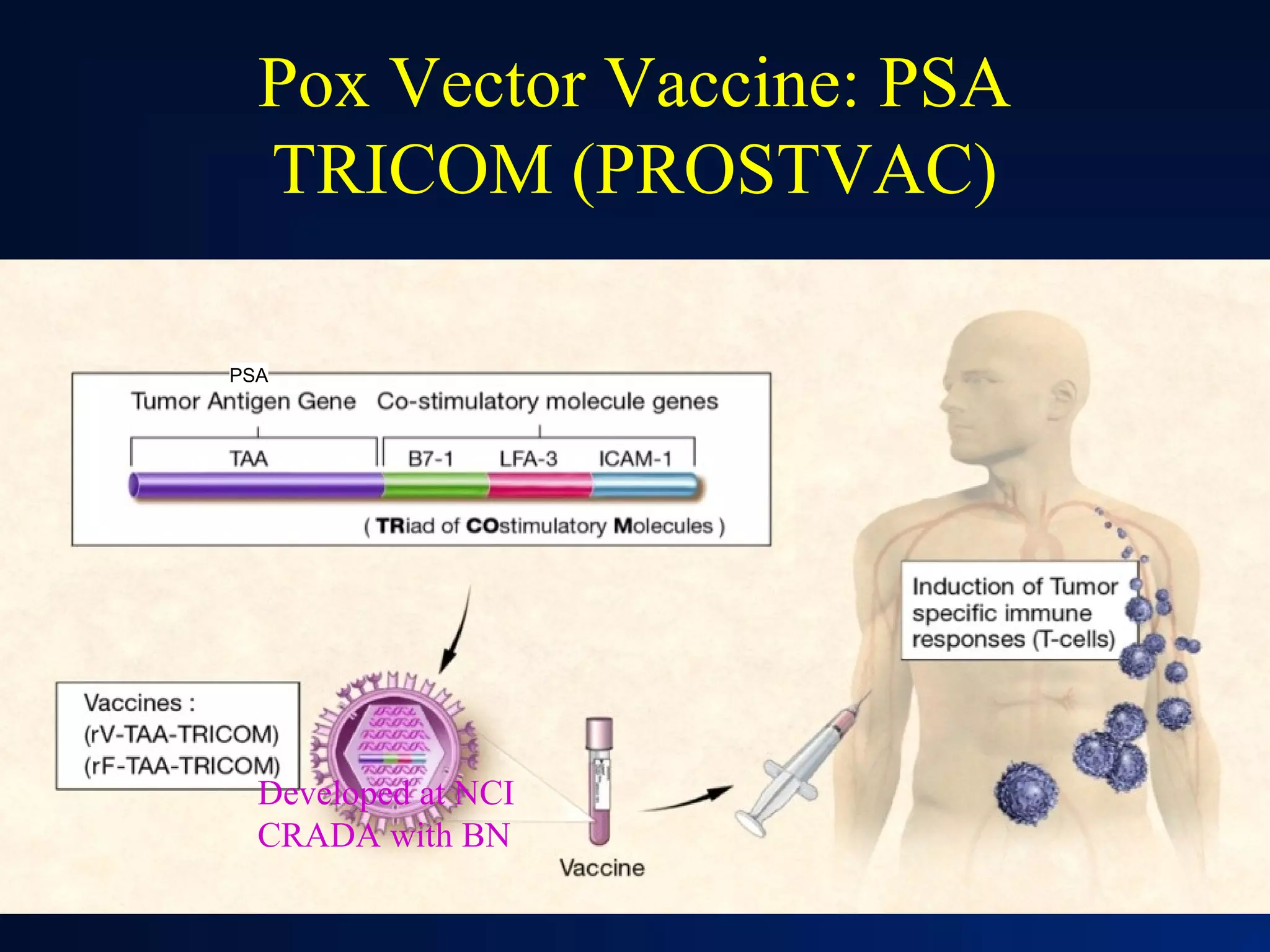
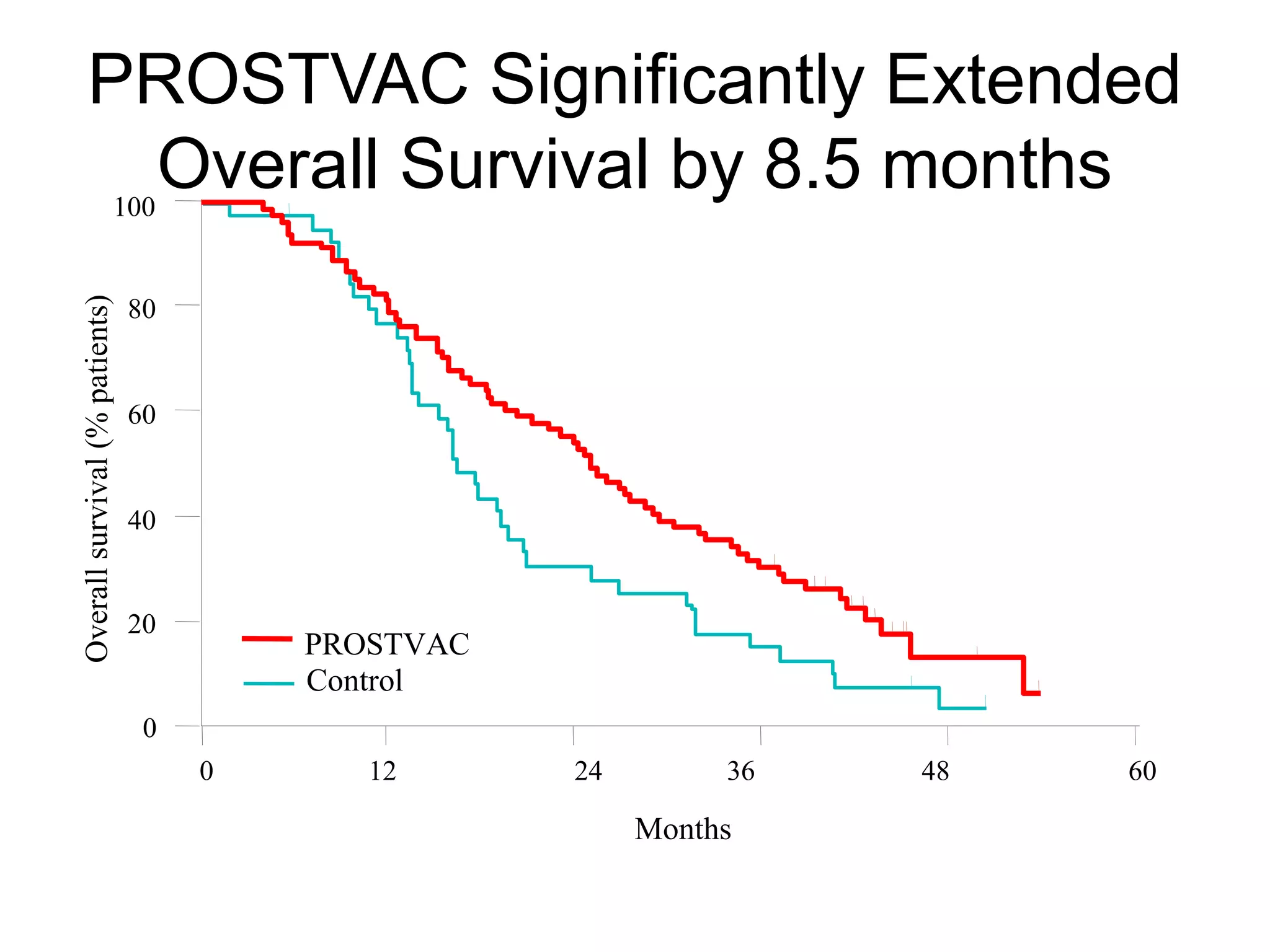
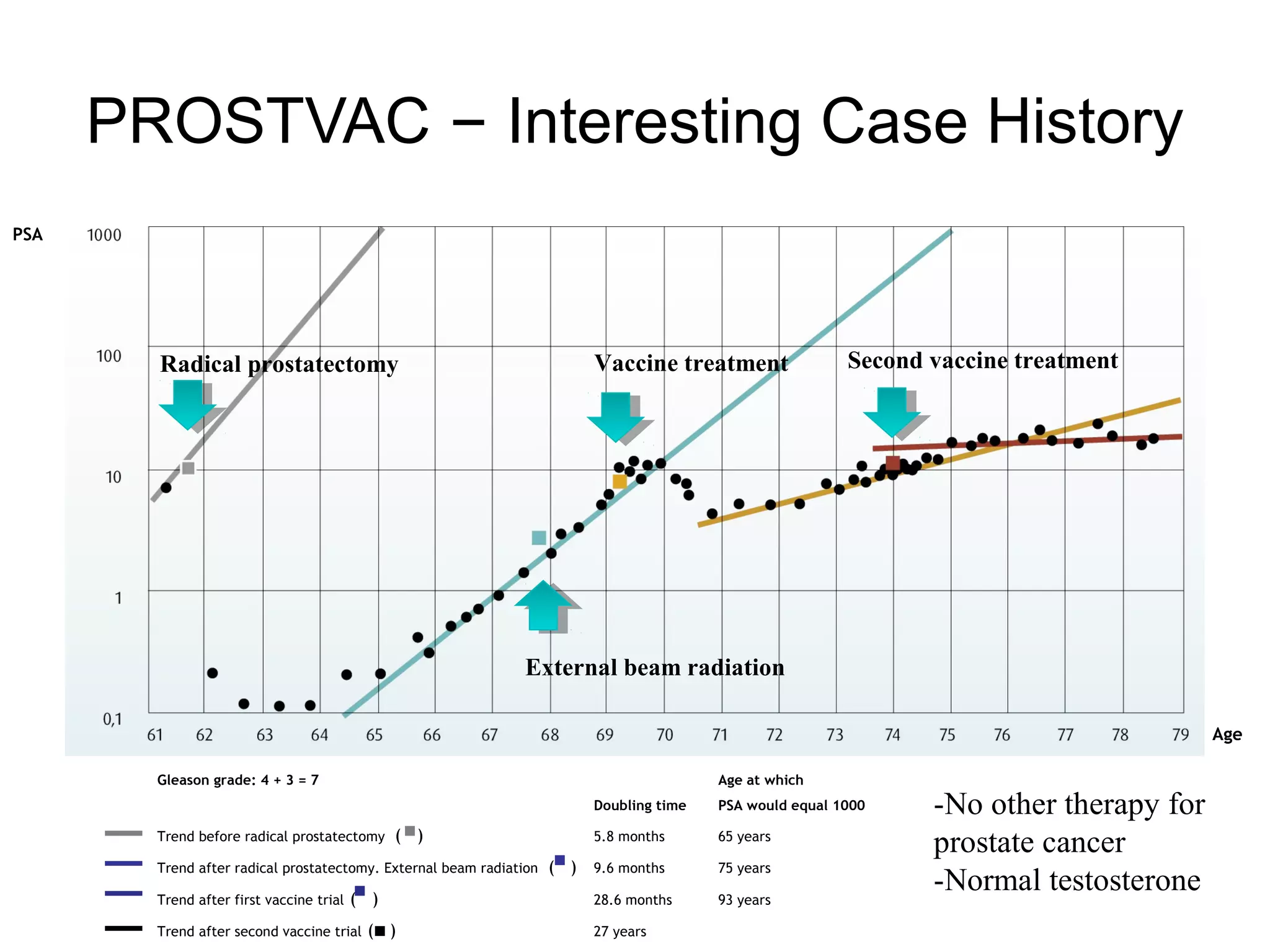
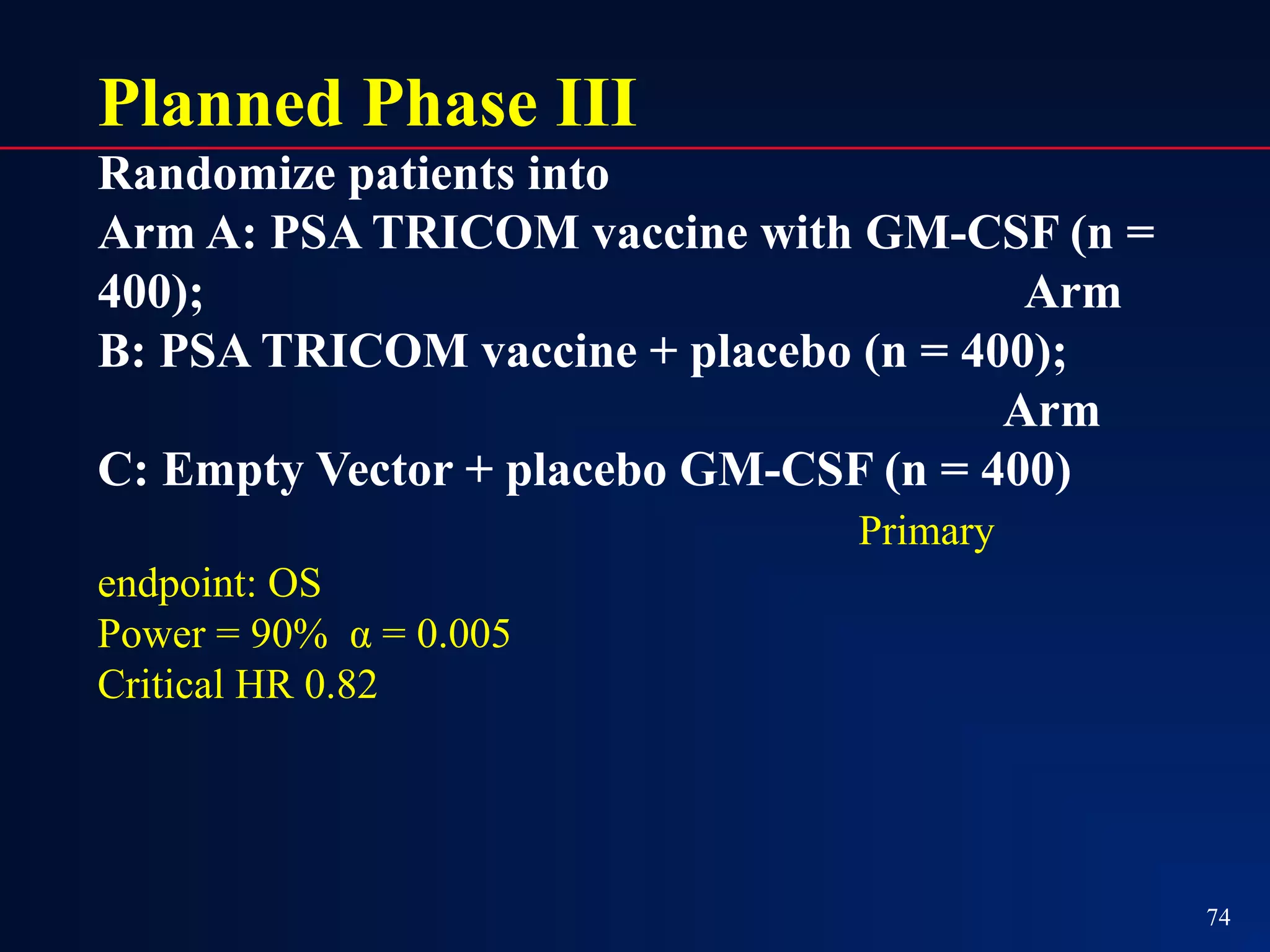
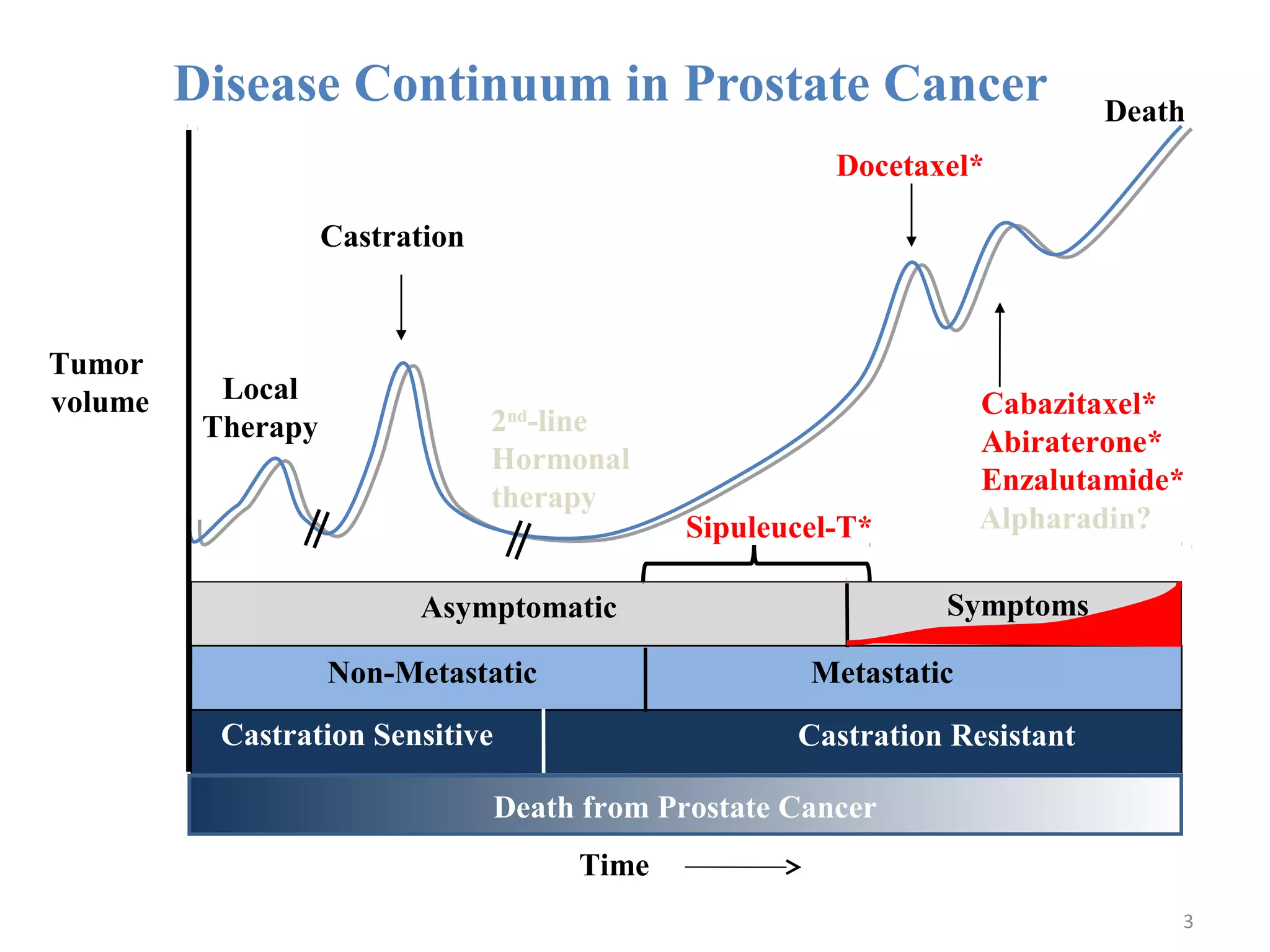
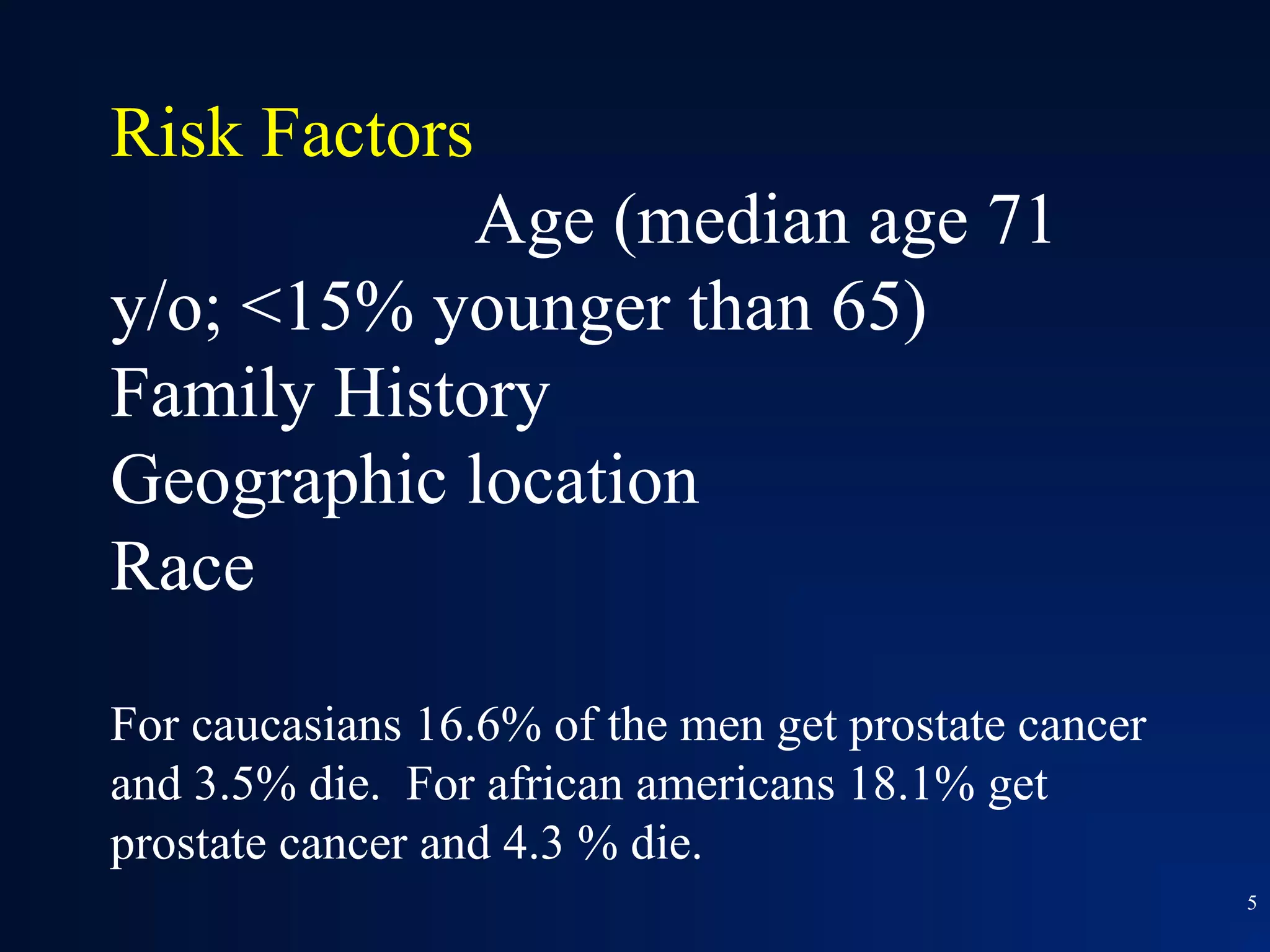
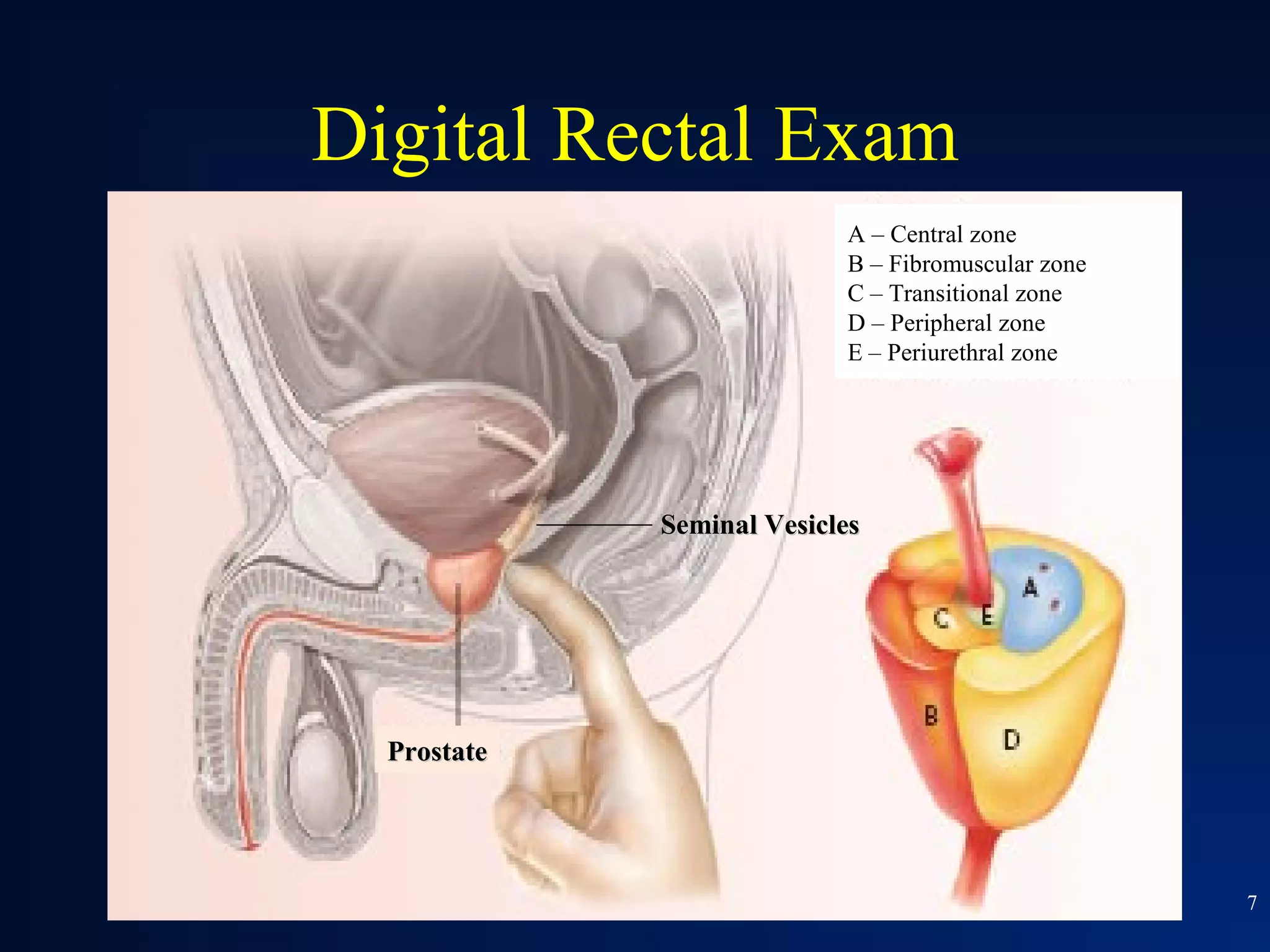
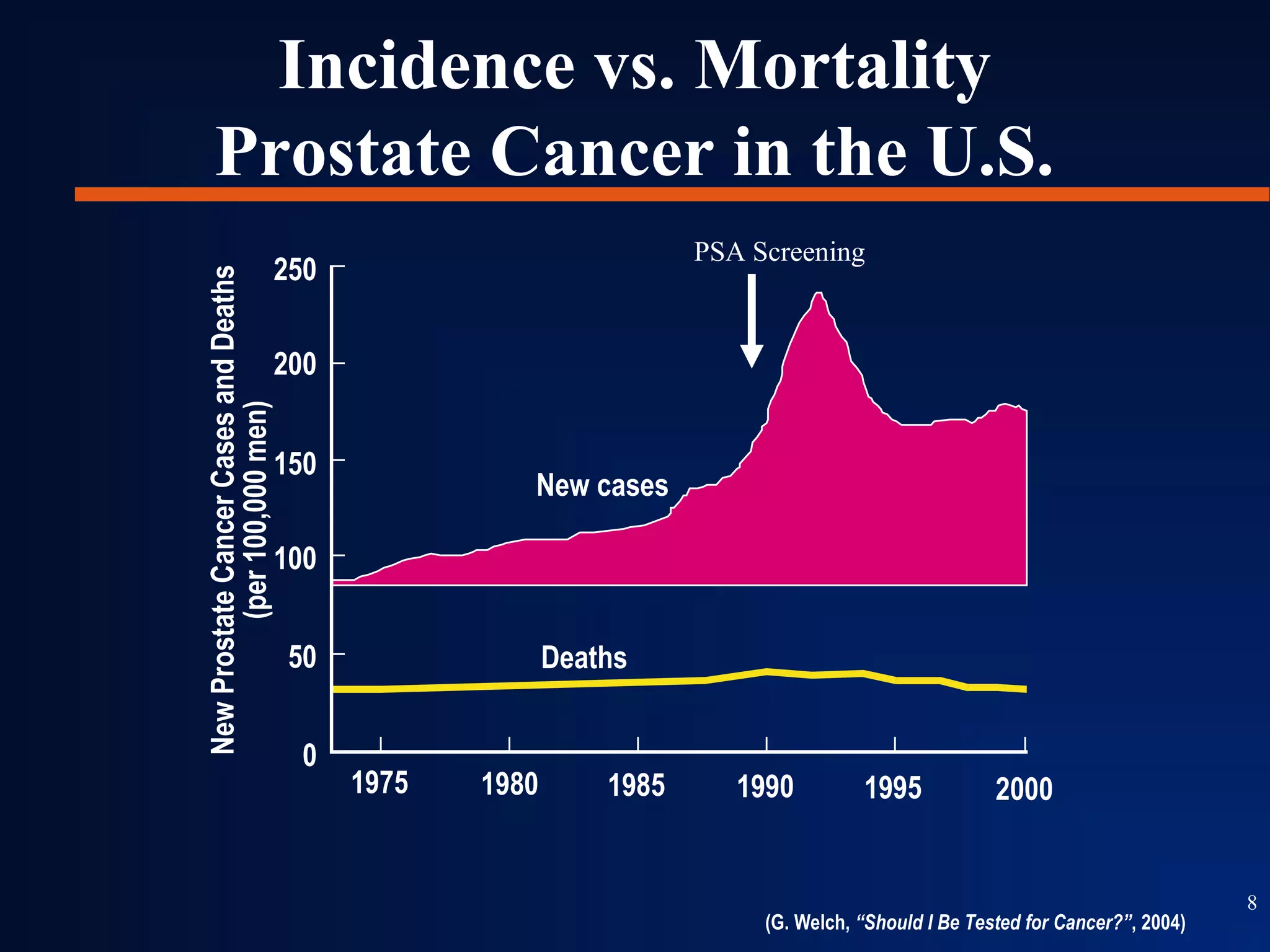
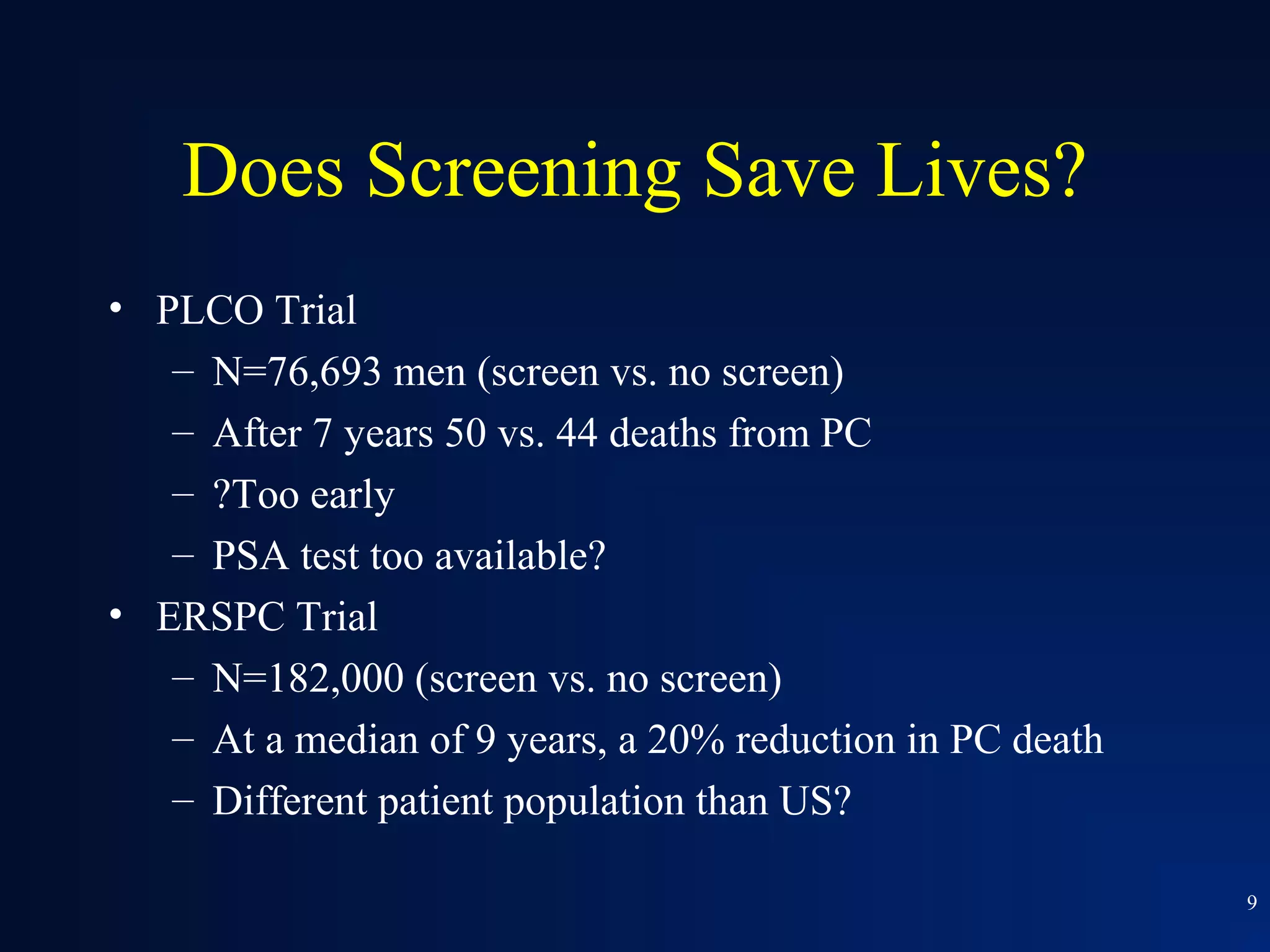
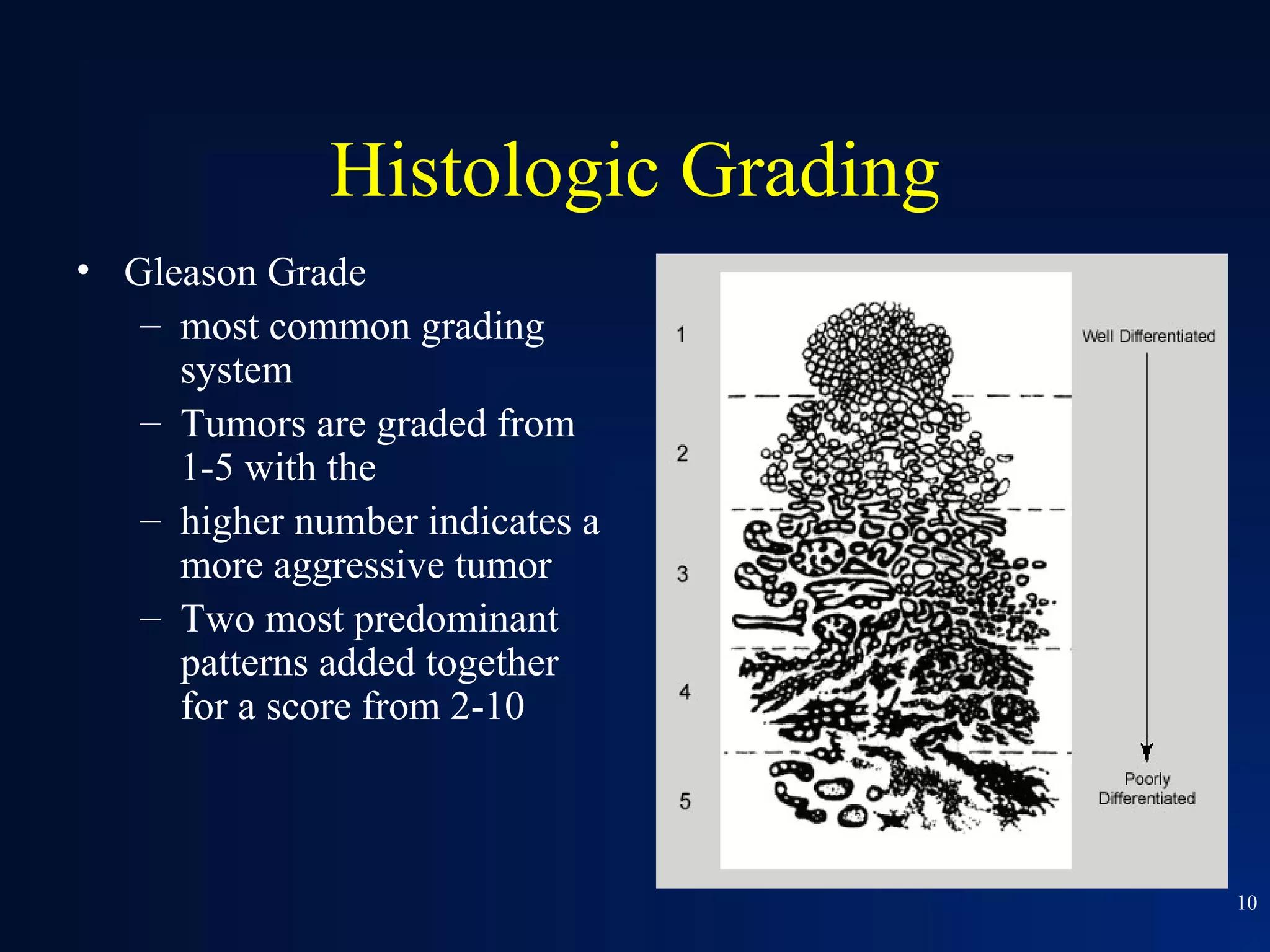
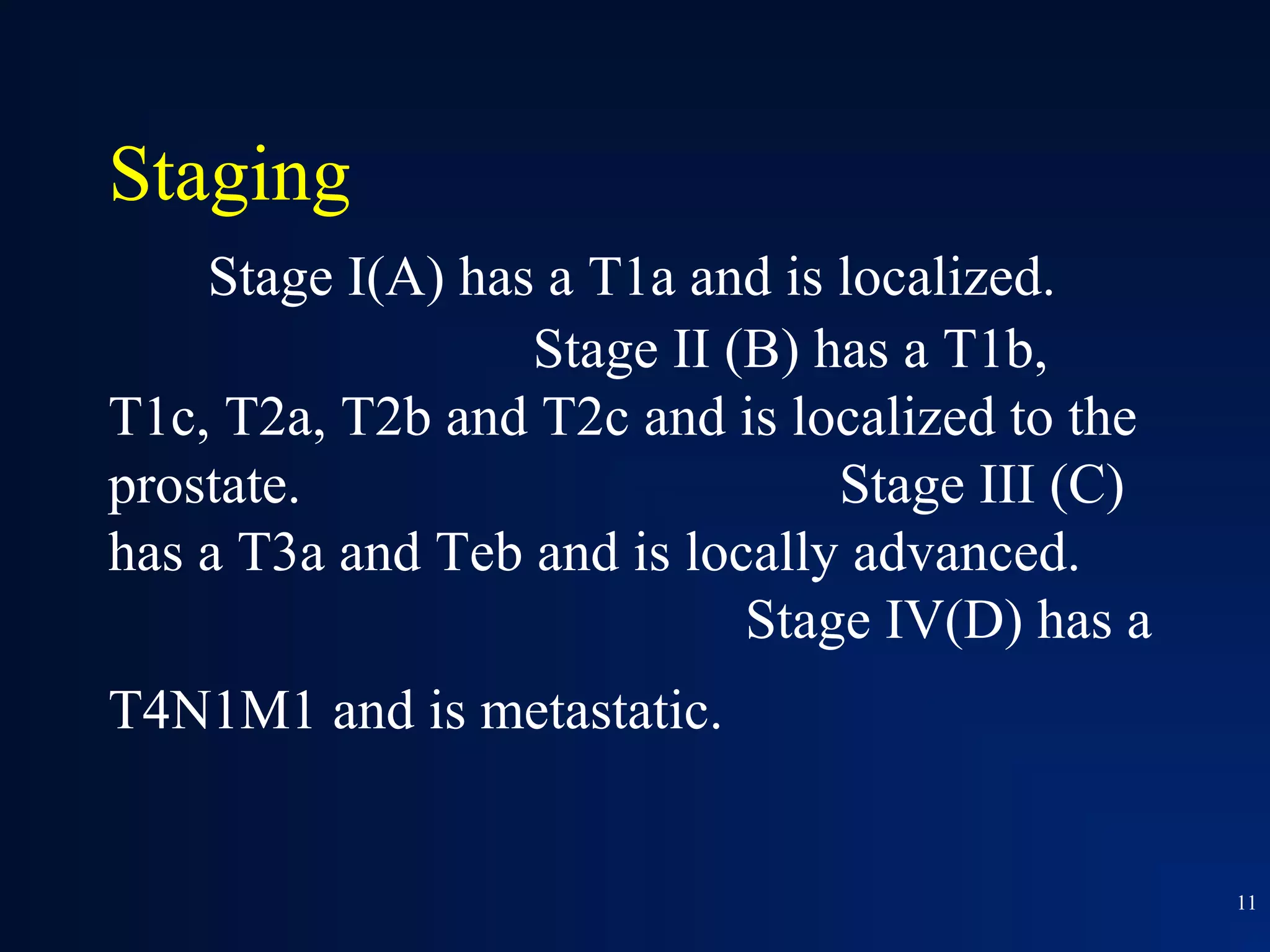
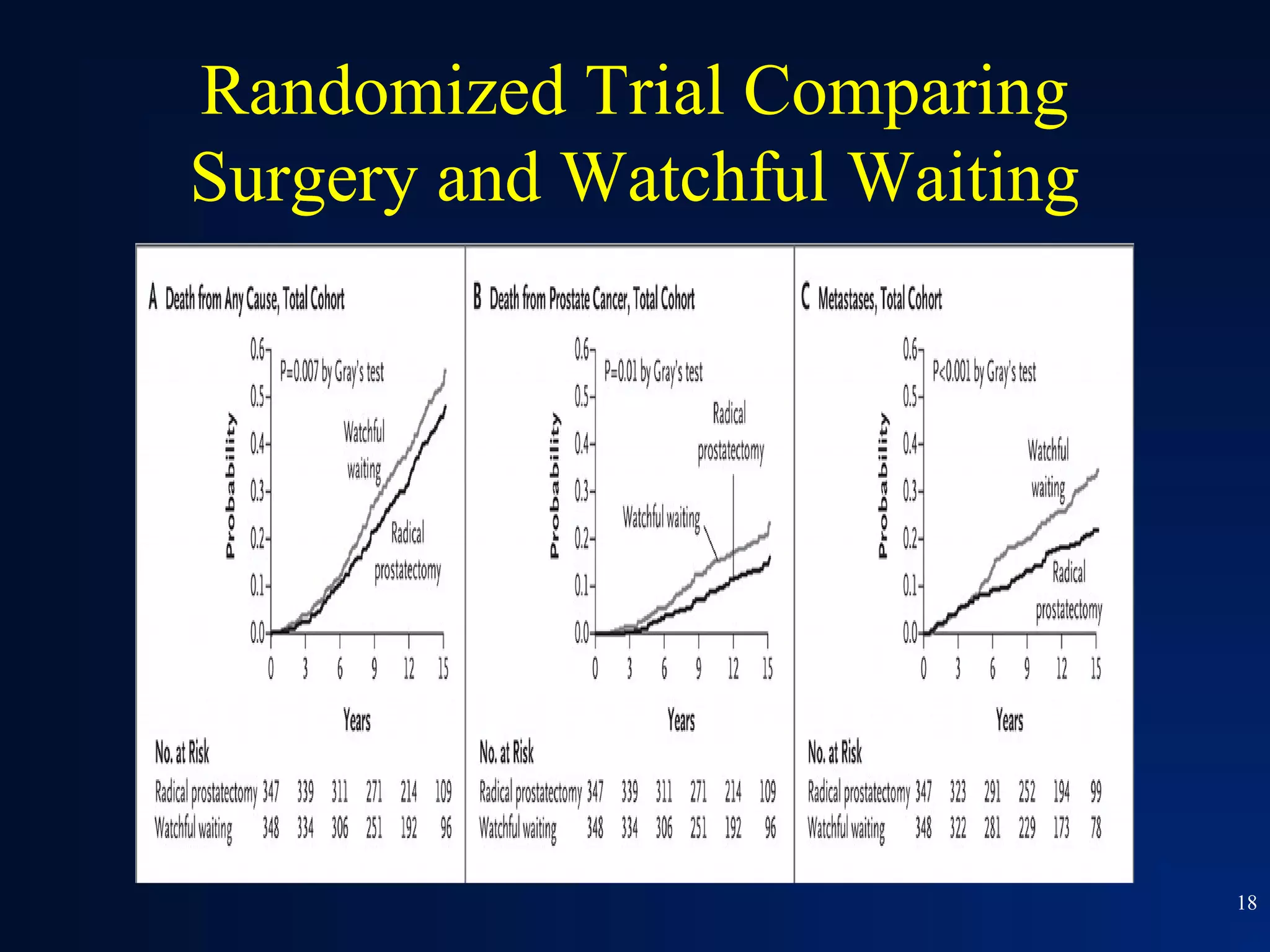
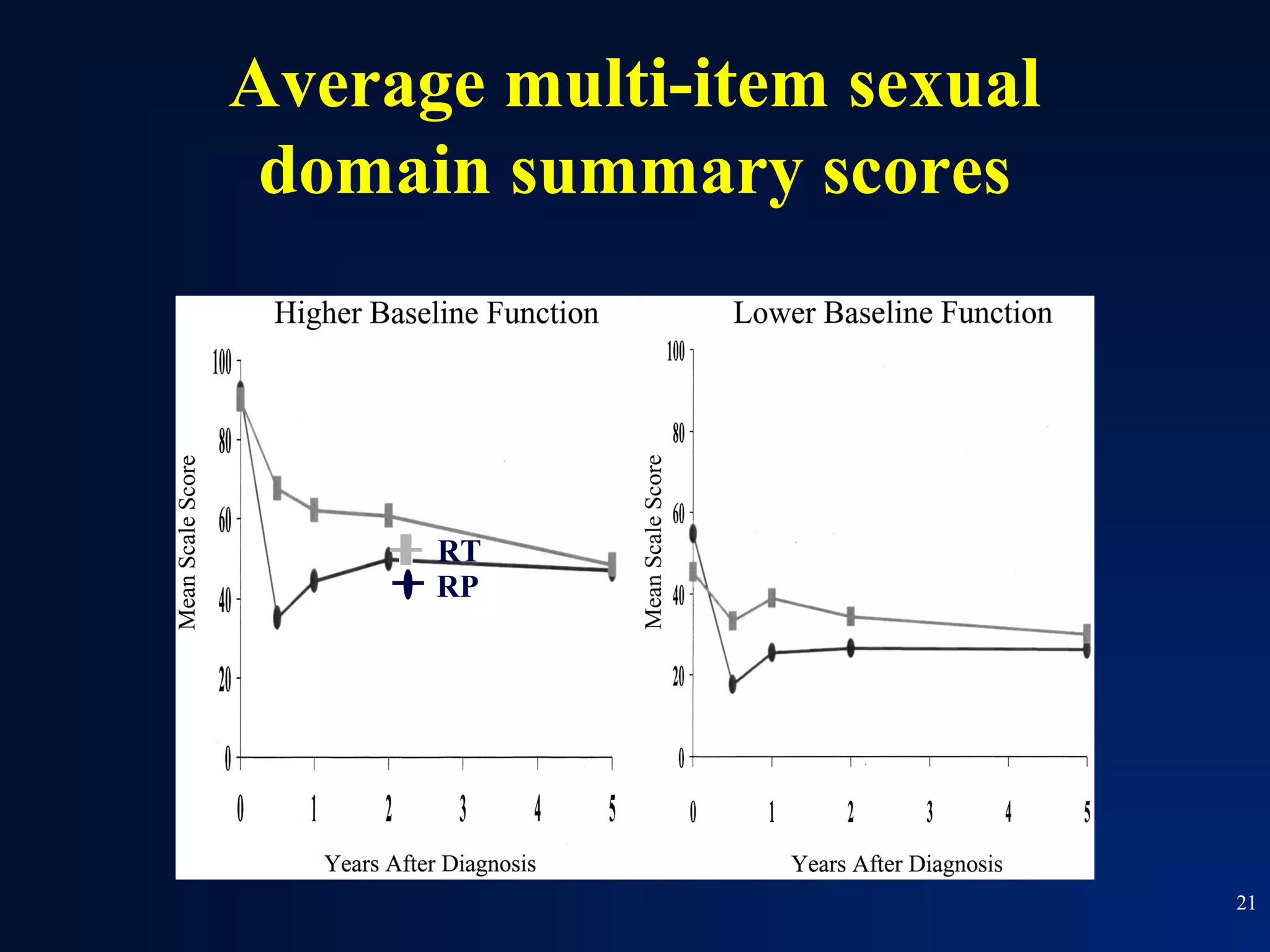
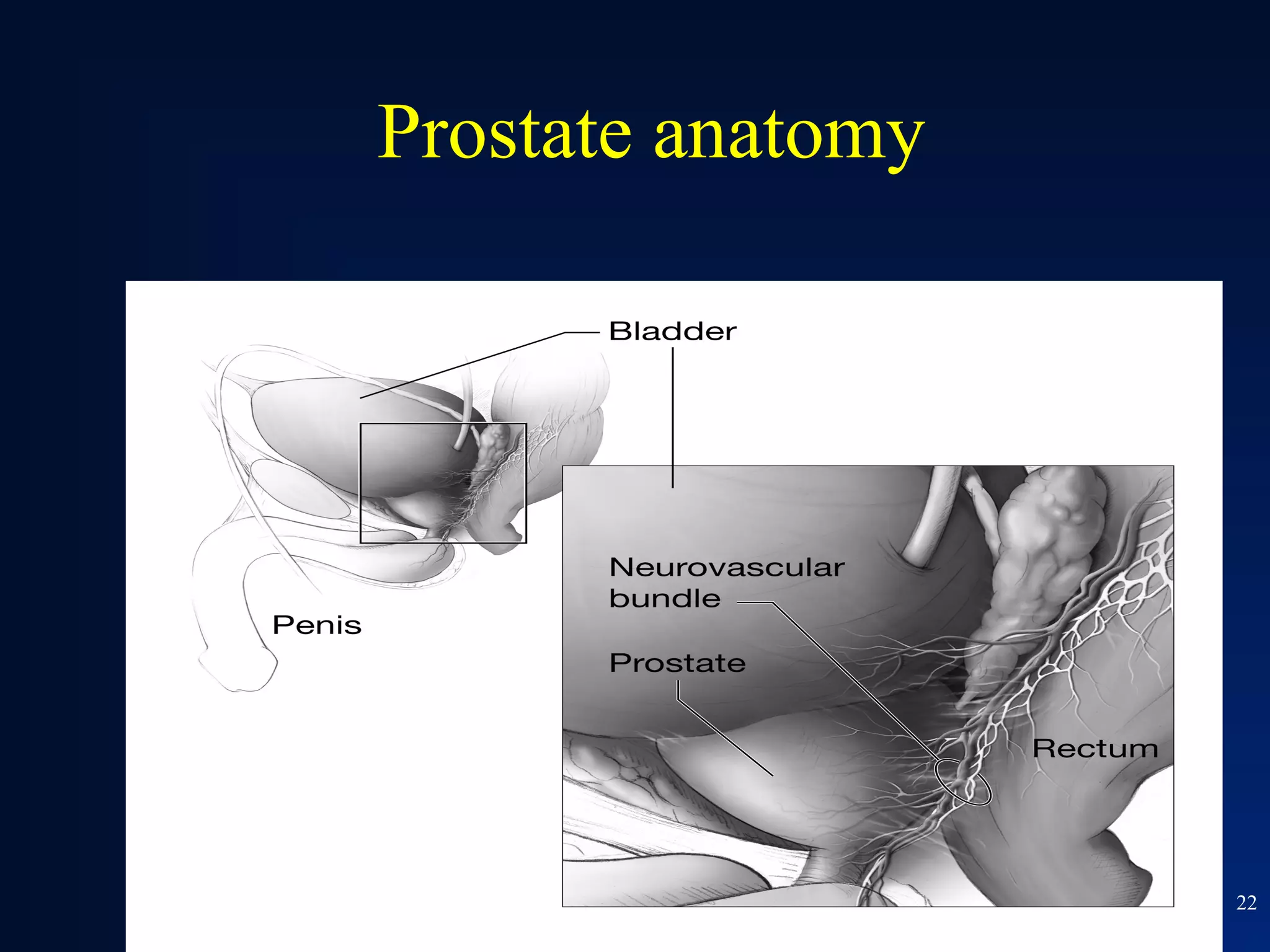
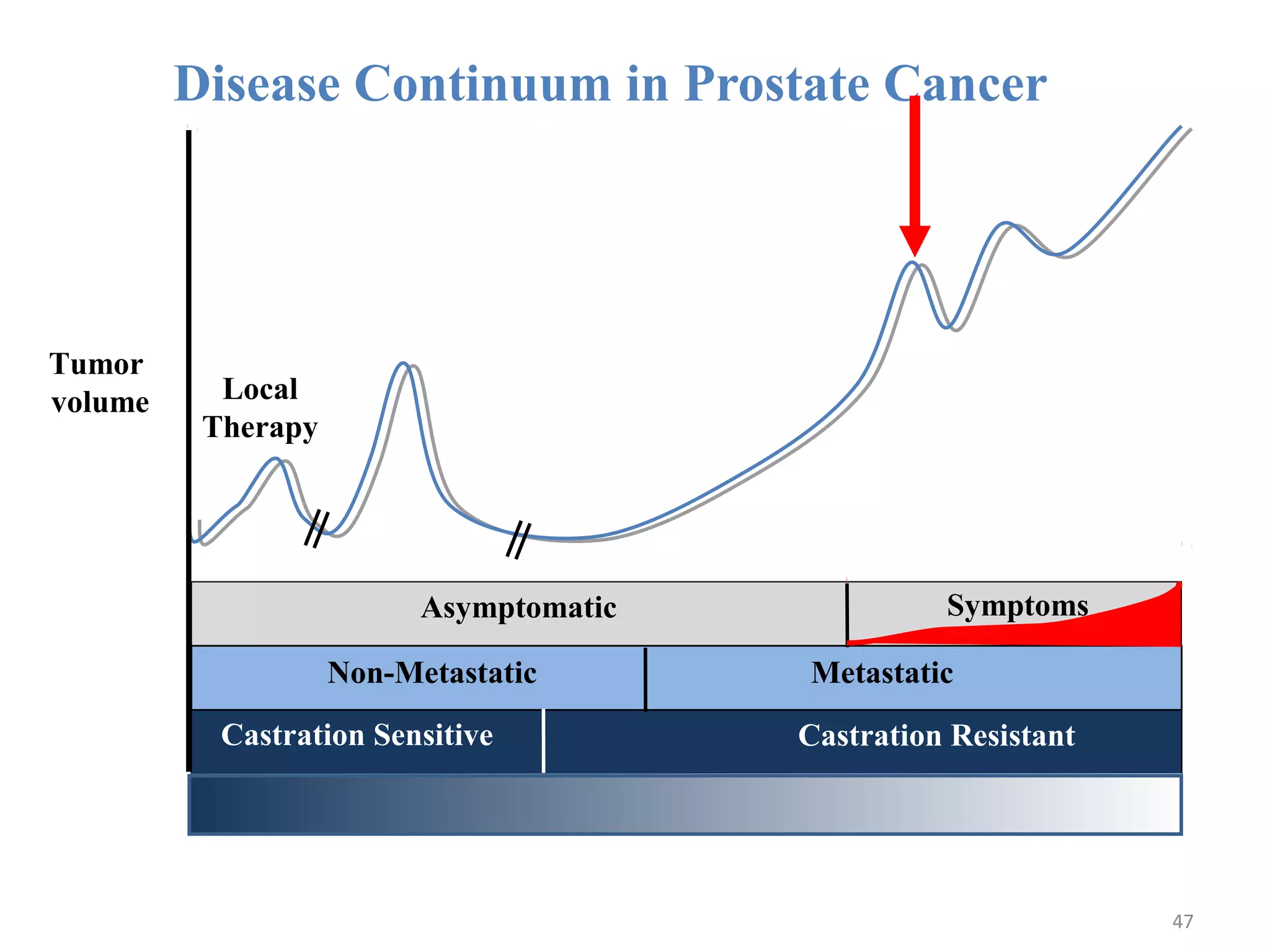
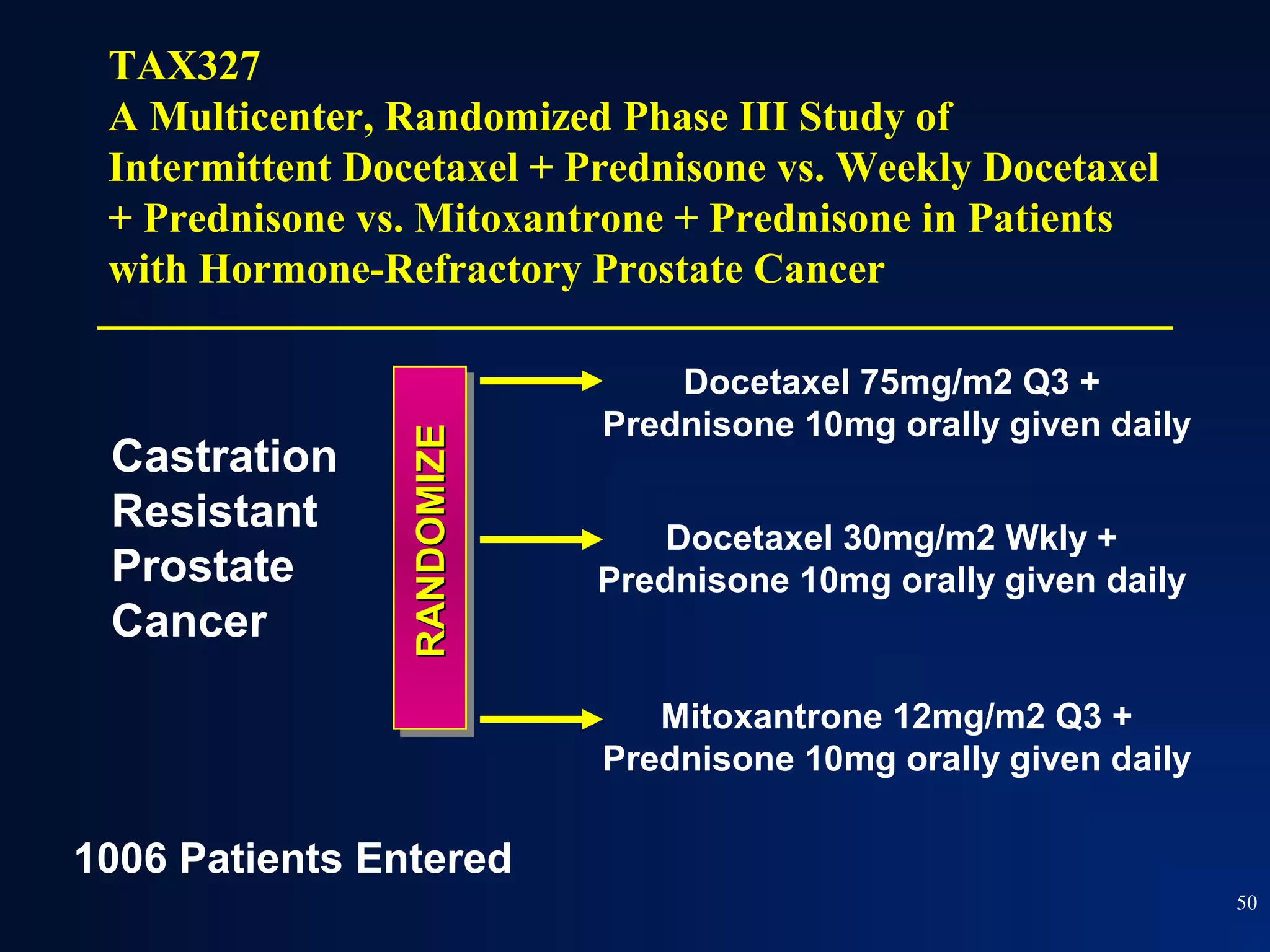
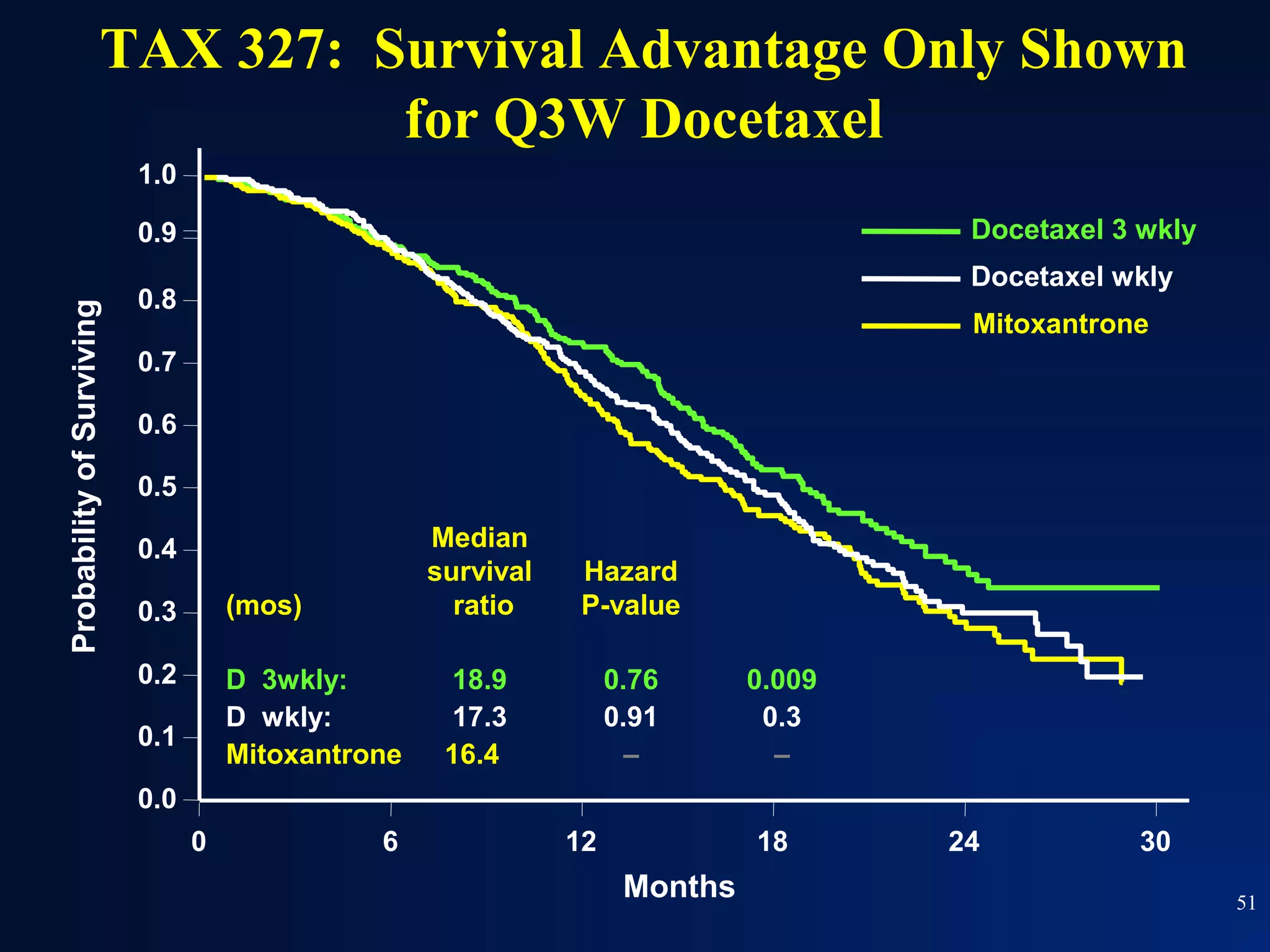
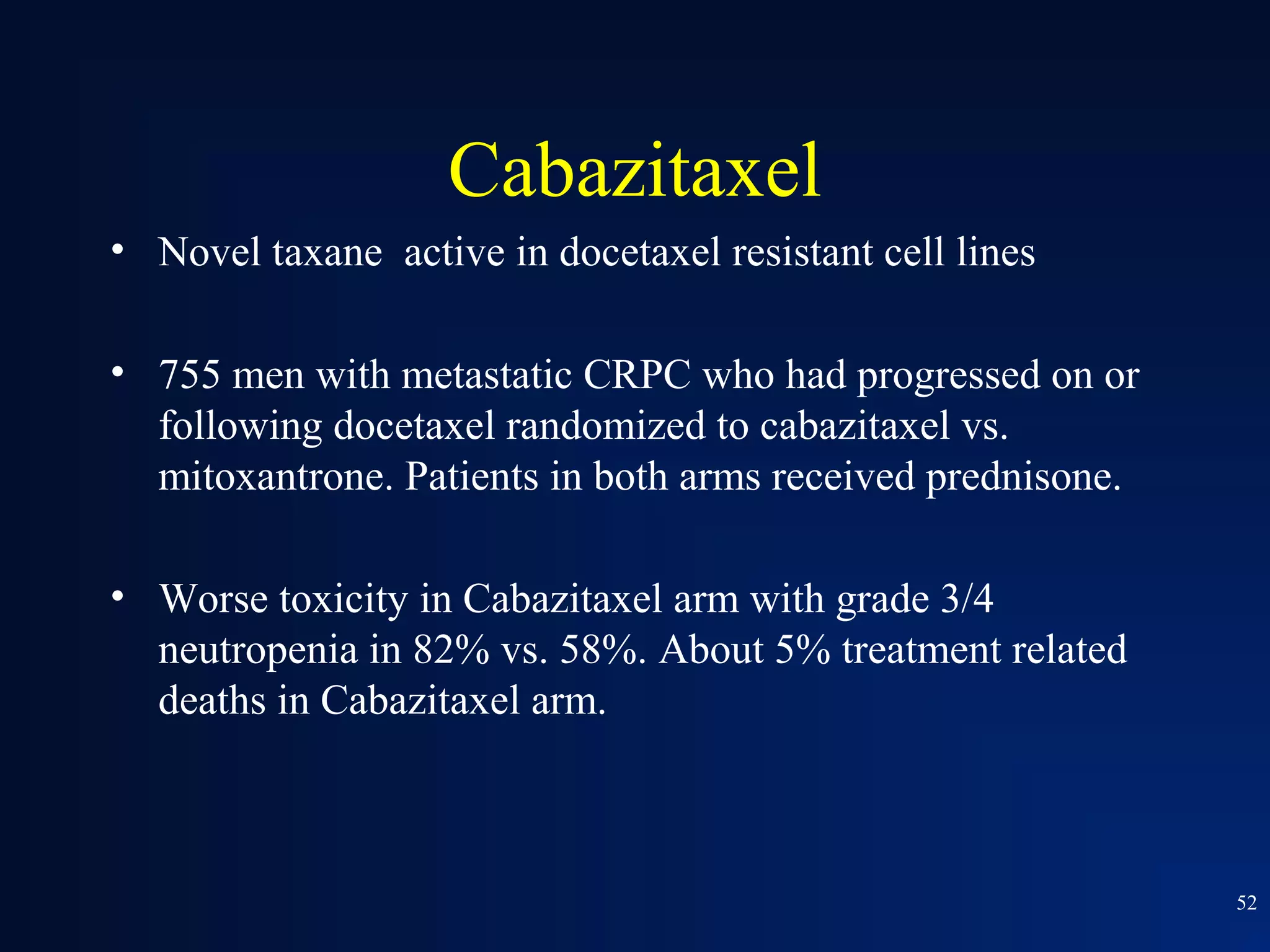
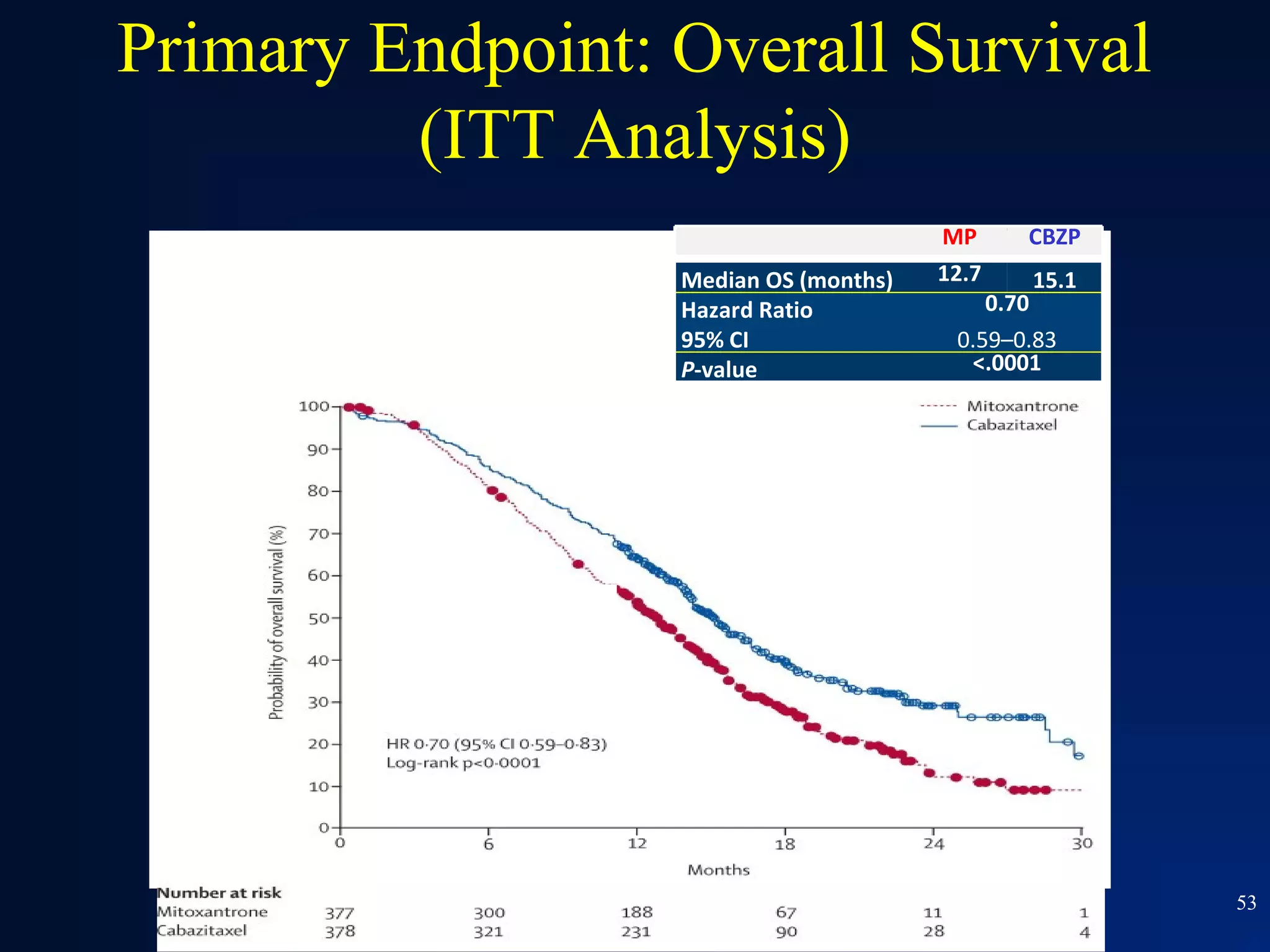
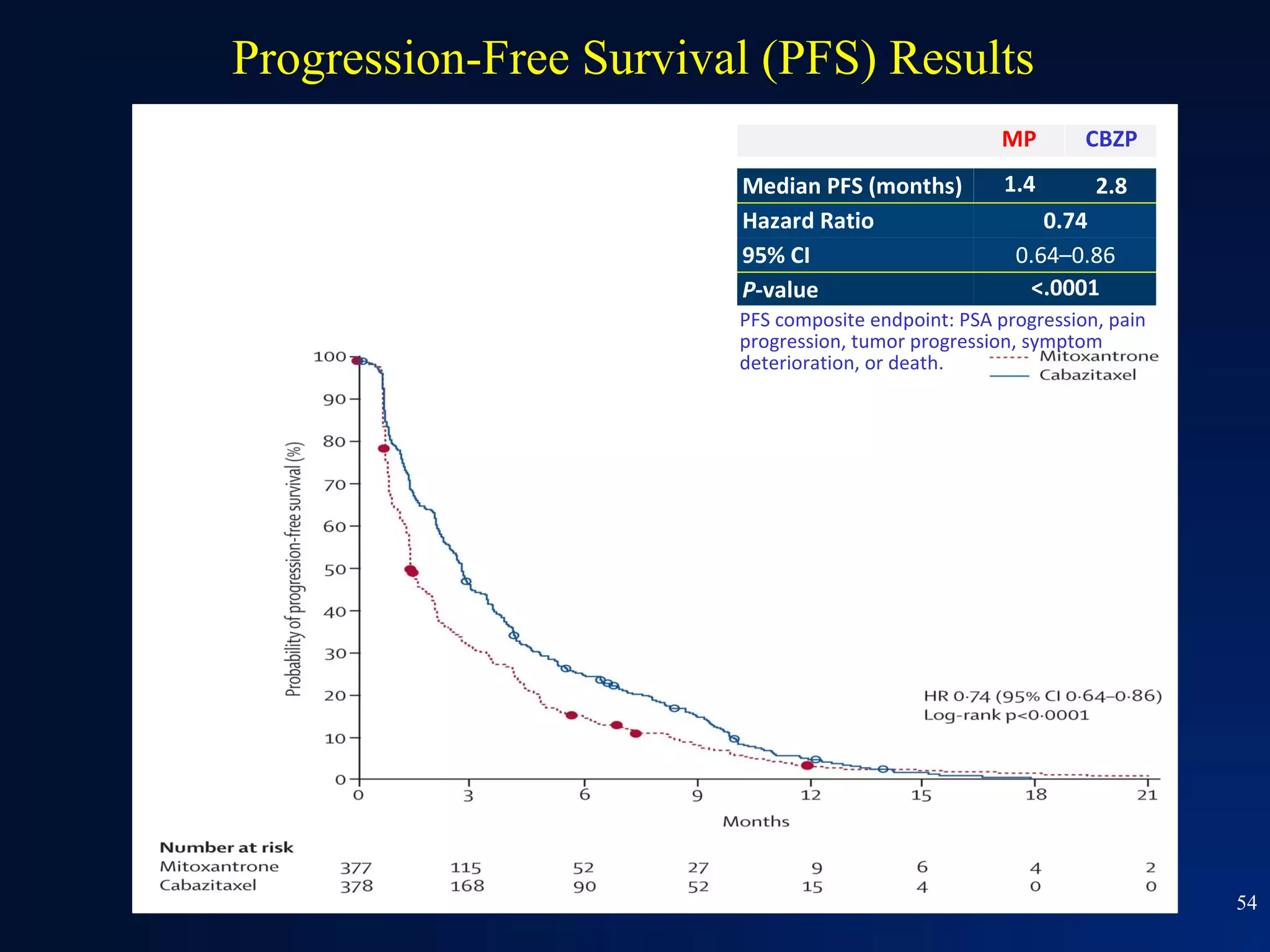
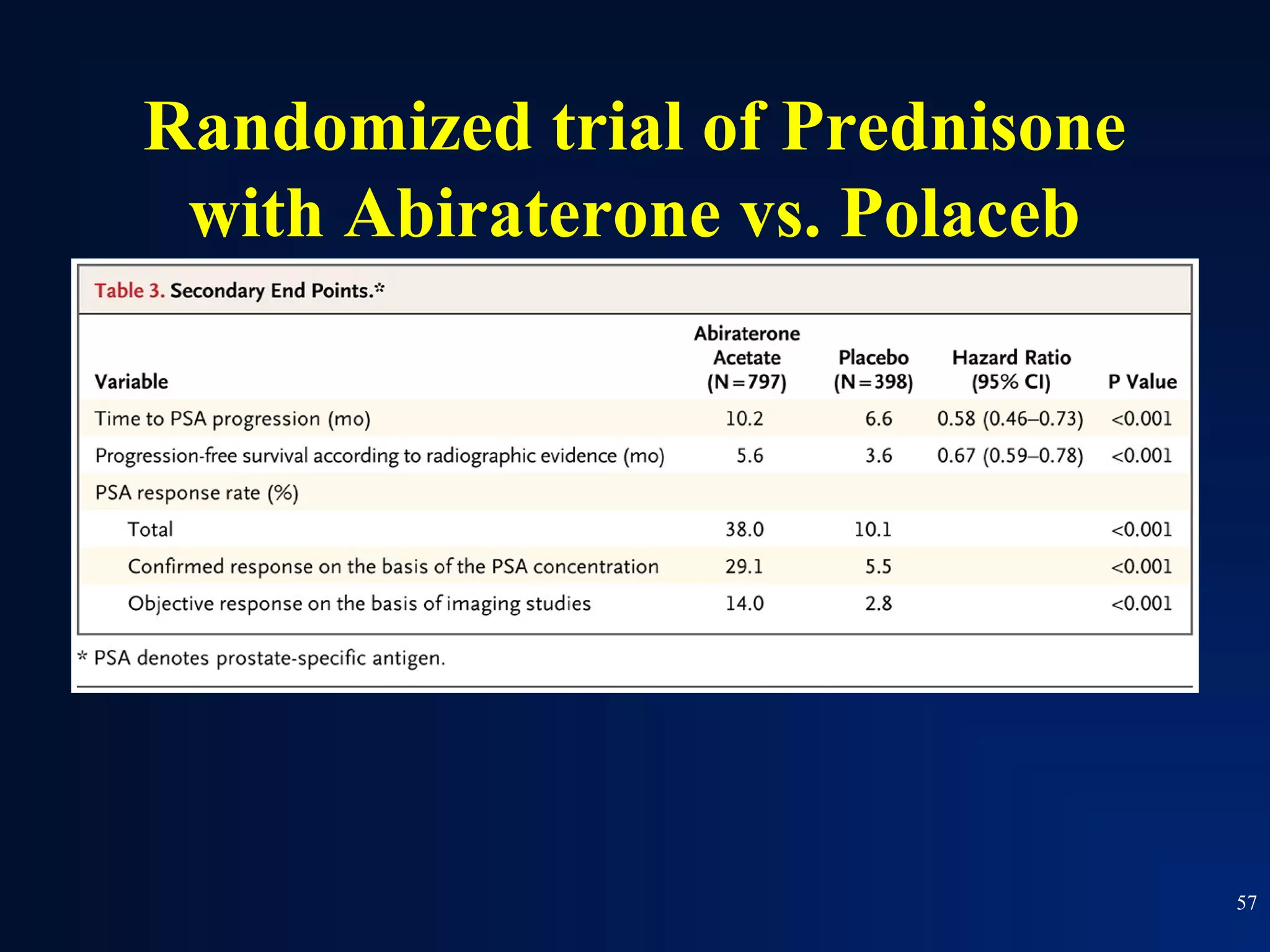
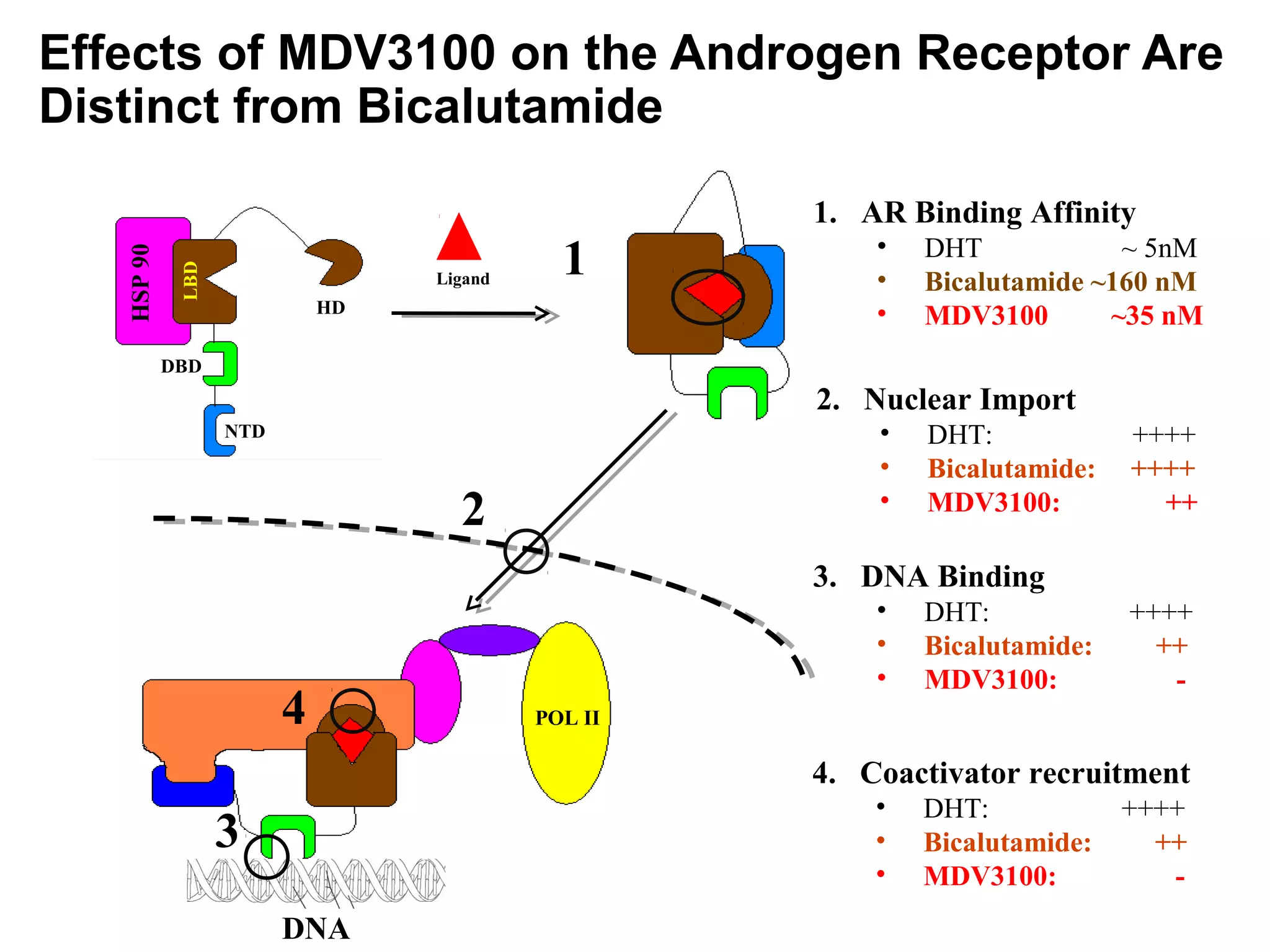
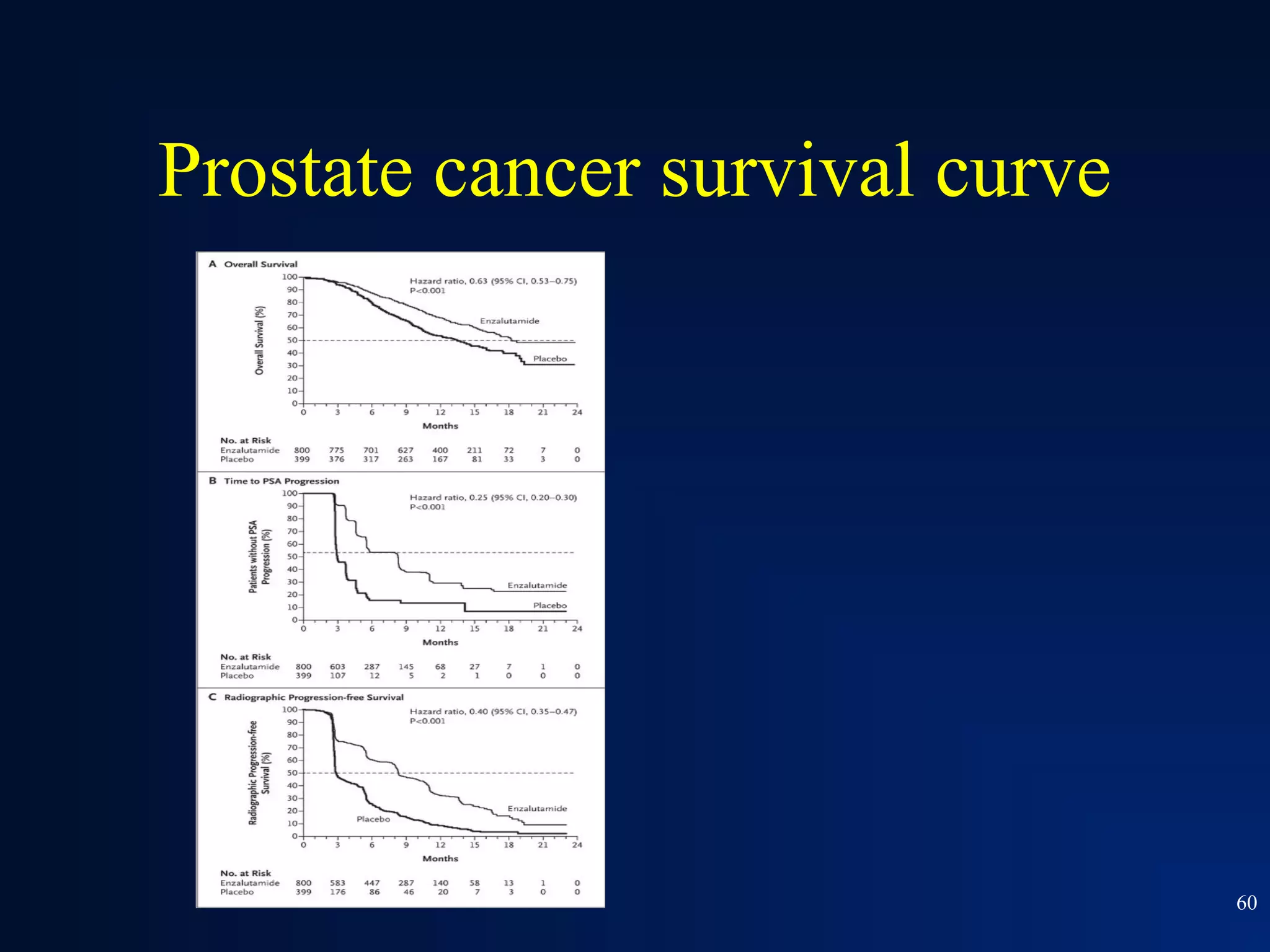
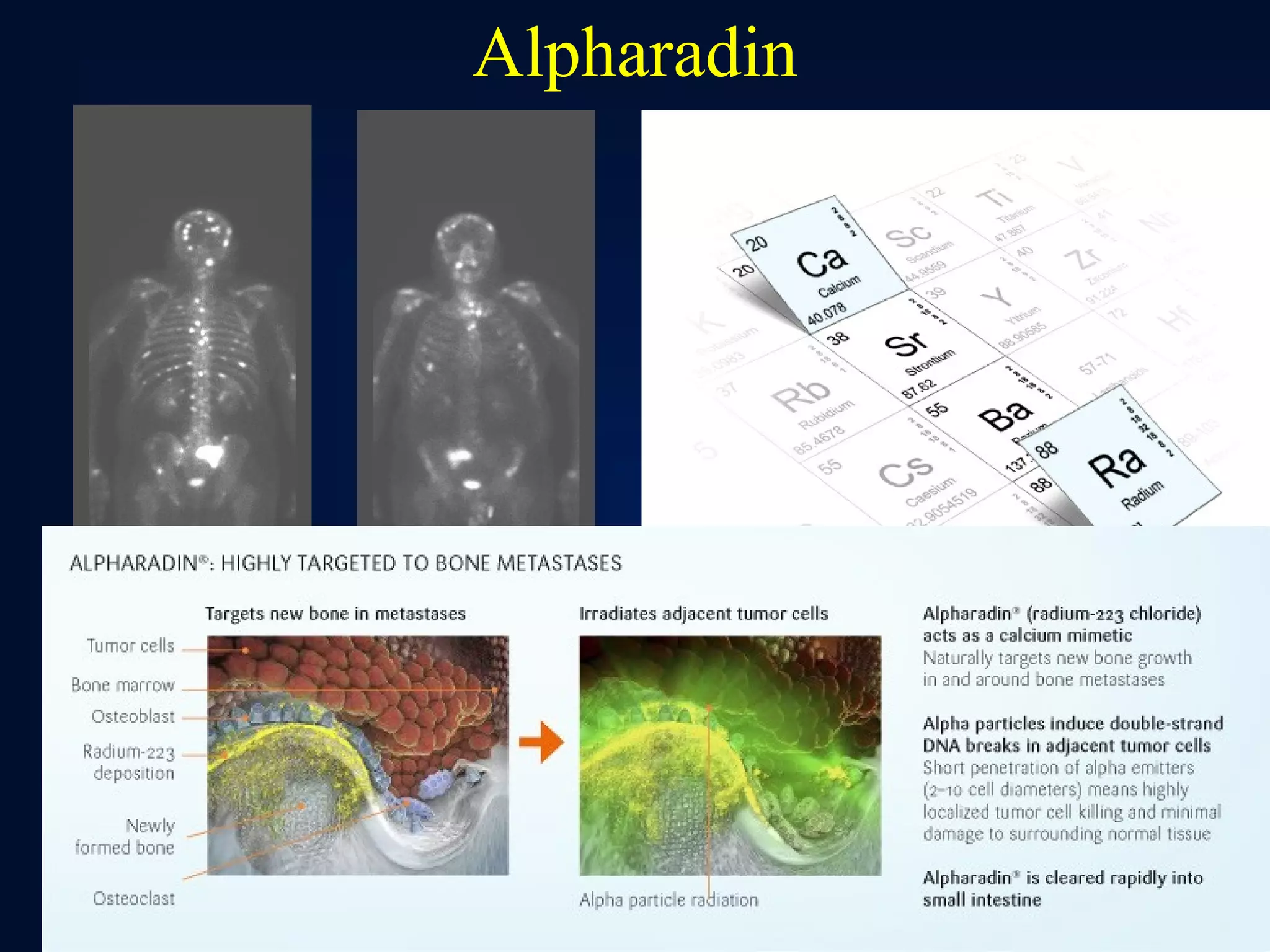
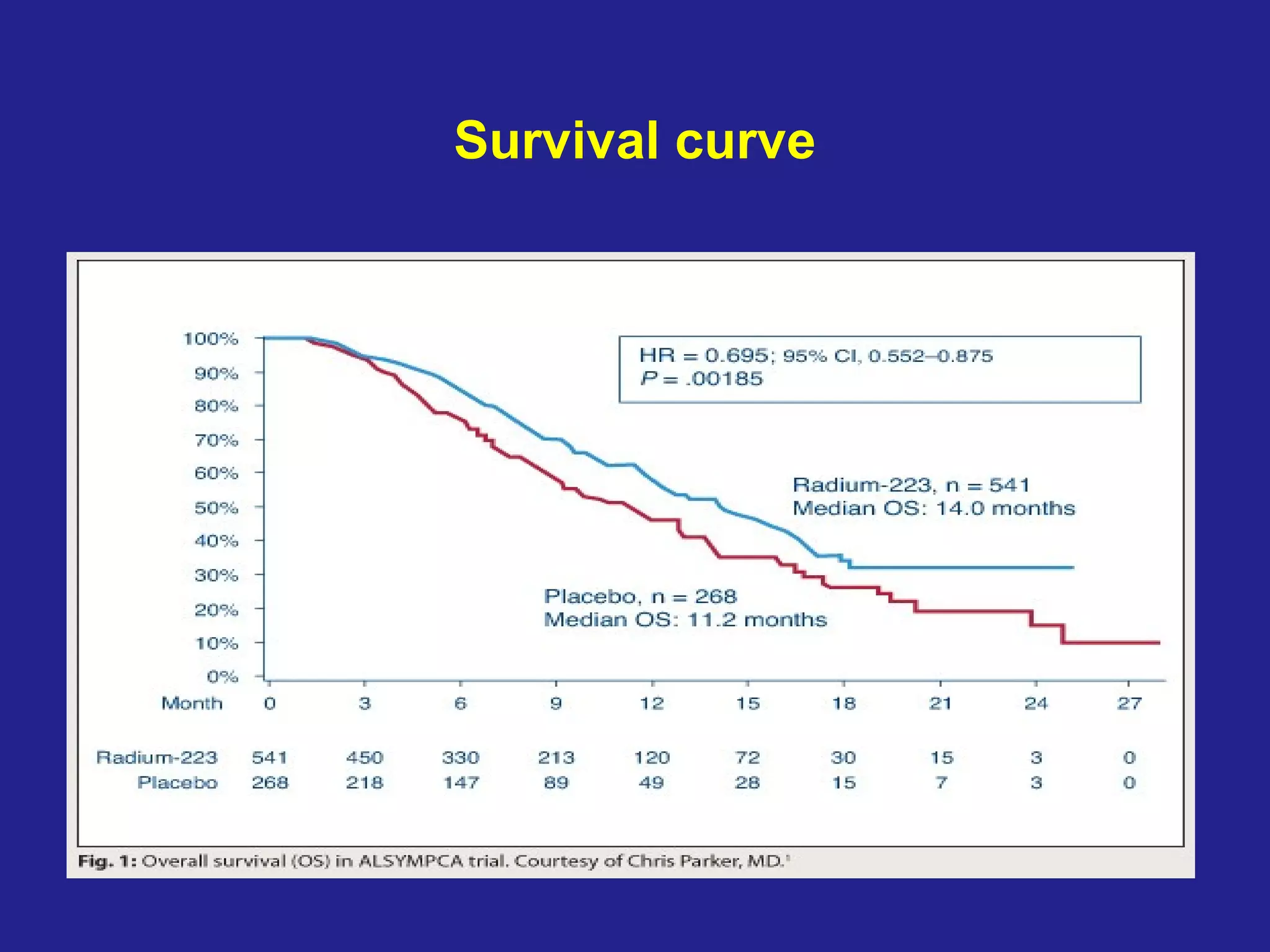
This document summarizes prostate cancer detection, prognosis, treatment options, and future directions. It discusses standard therapies including surgery, radiation, hormone therapy, chemotherapy, and bone-targeted therapy. Emerging immunotherapies and clinical trials of new agents such as abiraterone and MDV3100 are also reviewed. The disease continuum moves from localized to advanced disease, and treatments aim to target different stages from non-metastatic to castration-resistant prostate cancer.







































![Sipuleucel-T: IMPACT Overall Survival:
Primary Endpoint Intent-to-Treat Population
100
P = 0.032 (Cox model)
75
HR = 0.775 [95% CI: 0.614, 0.979]
Percent Survival
Median Survival Benefit = 4.1 Mos.
50 Sipuleucel-T (n = 341)
Median Survival: 25.8 Mos.
25
Placebo (n = 171)
Median Survival: 21.7 Mos.
0
0 6 12 18 24 30 36 42 48 54 60 66
Survival (Months) 66](https://image.slidesharecdn.com/gulley508traco2012-121119025324-phpapp01/75/Prostate-cancer-66-2048.jpg)