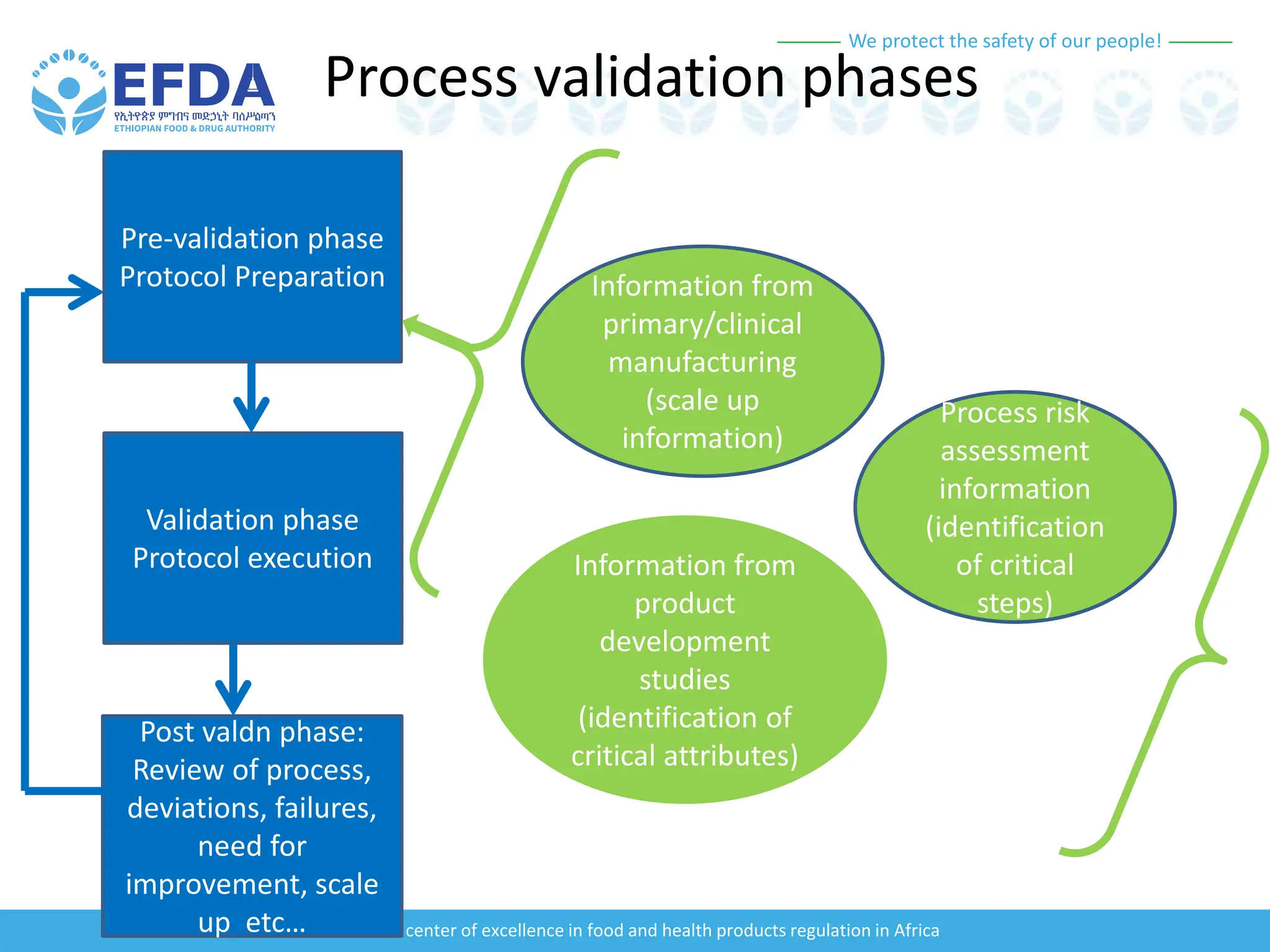
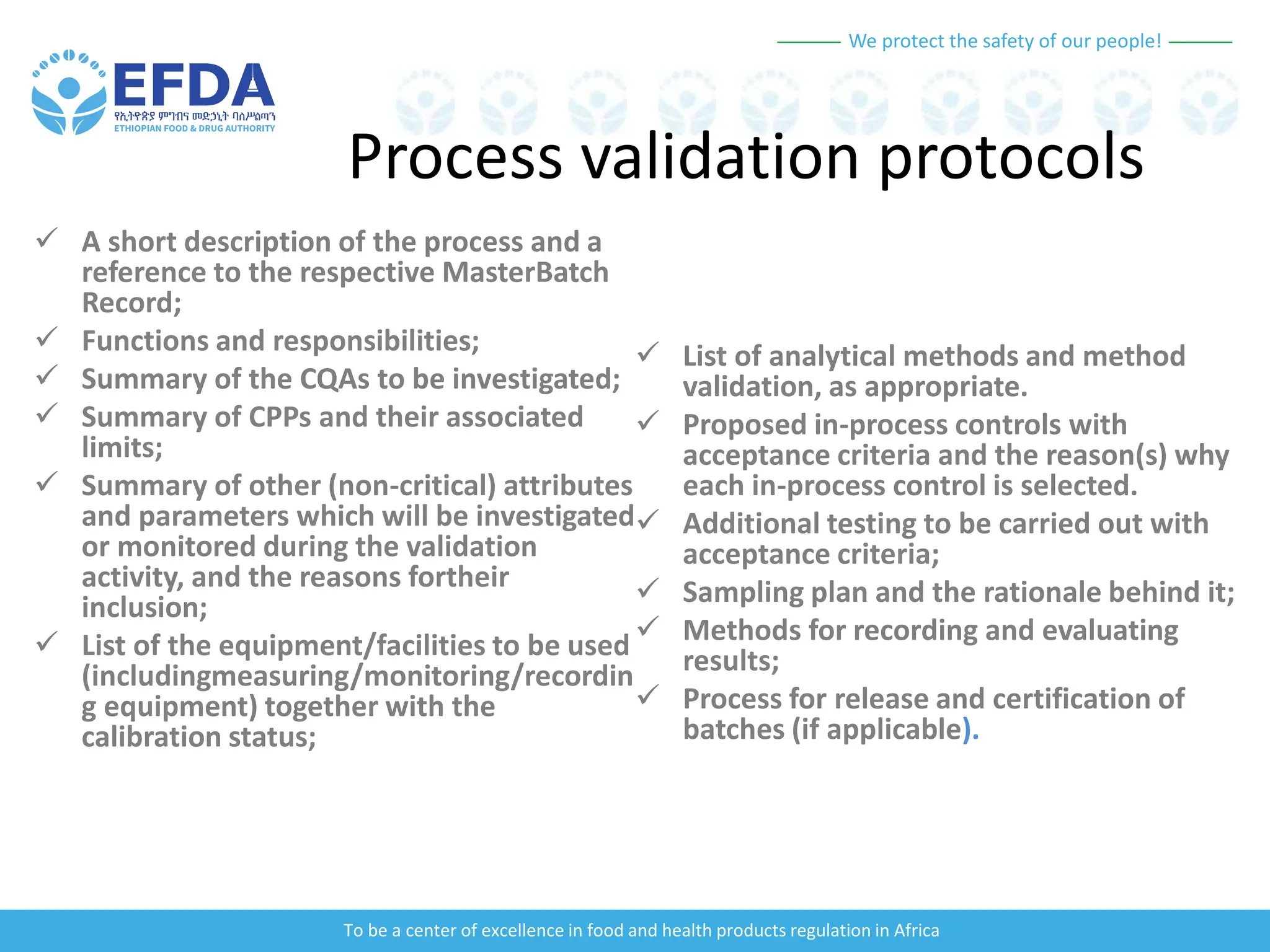
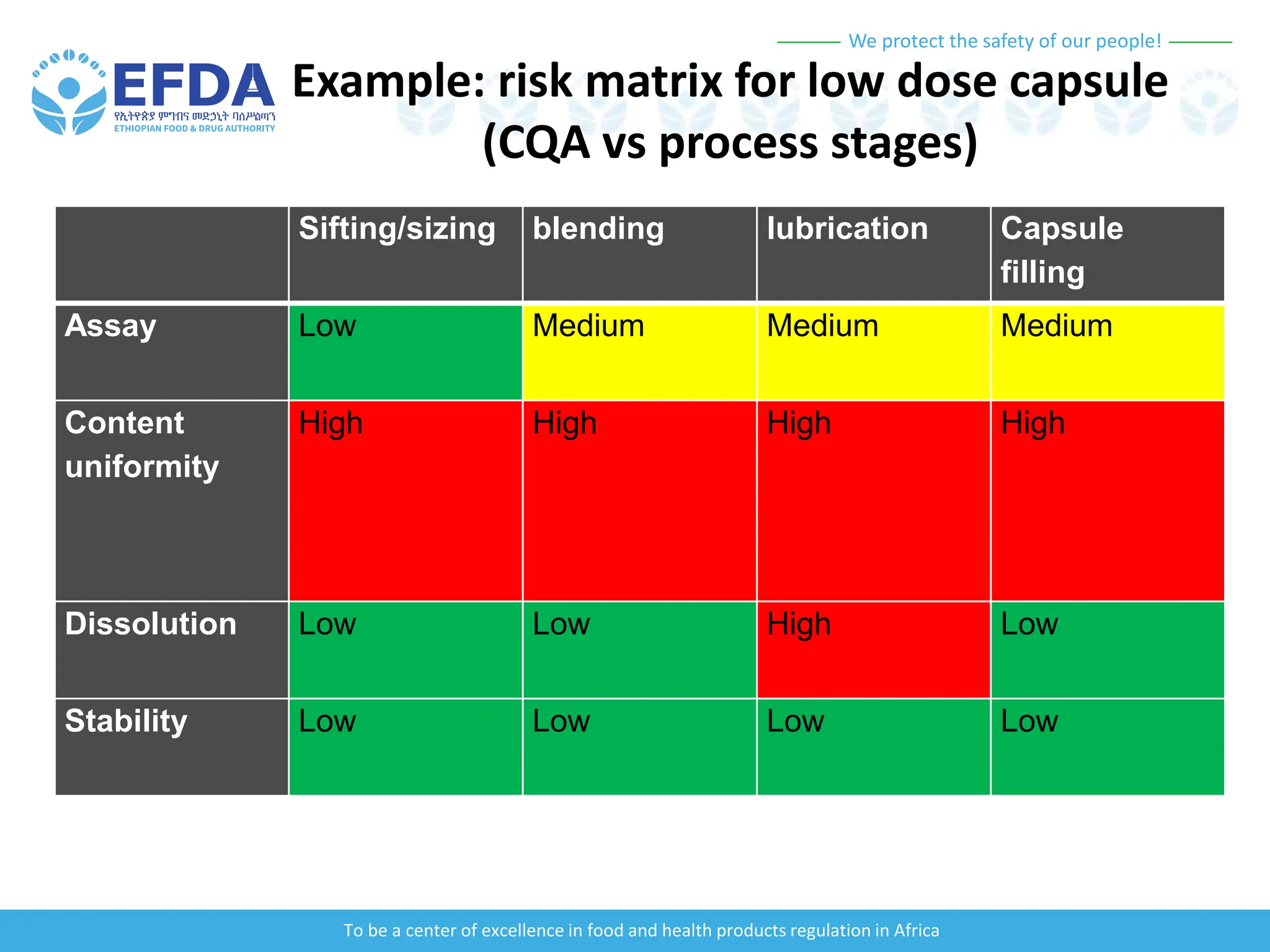
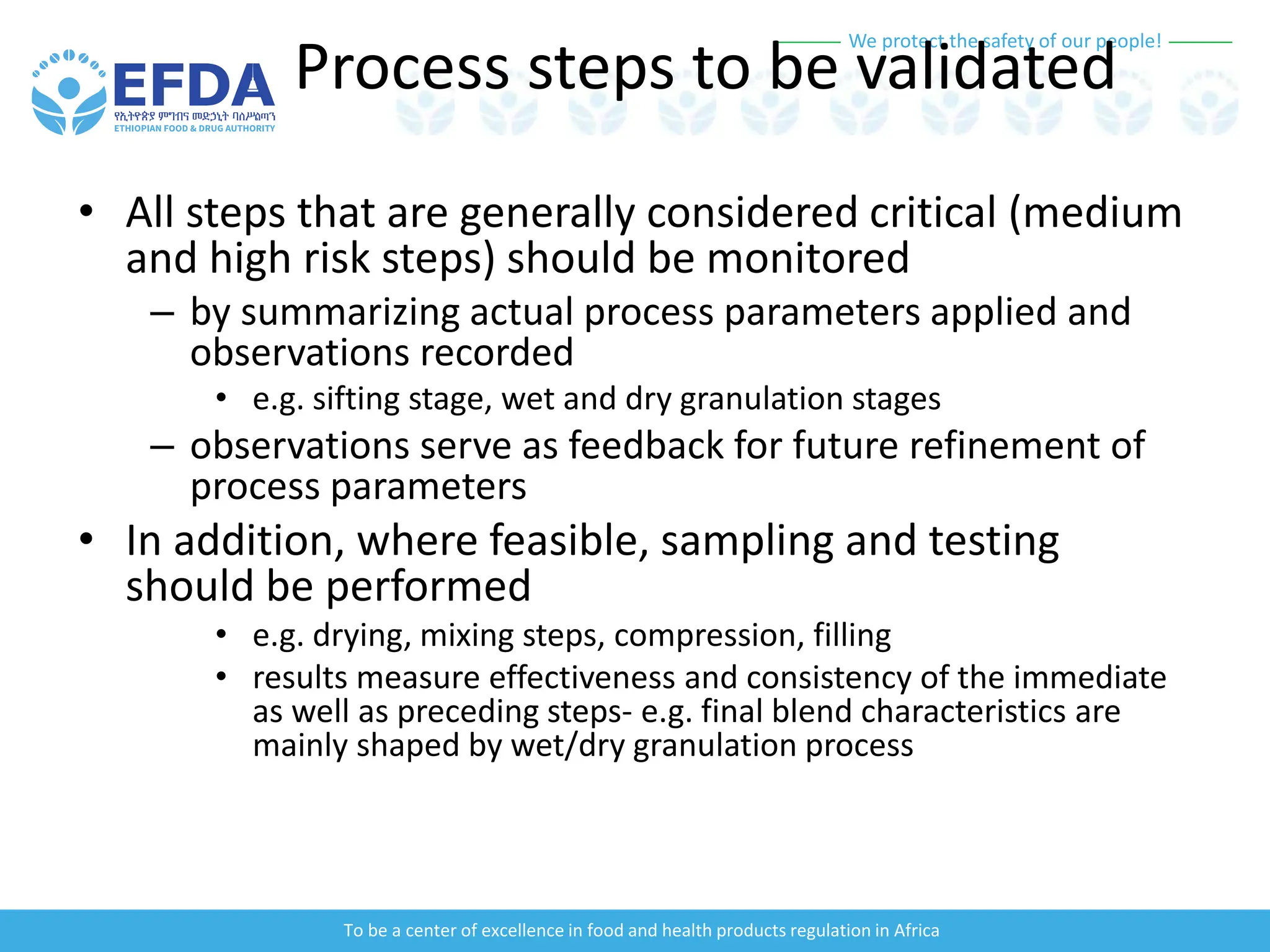
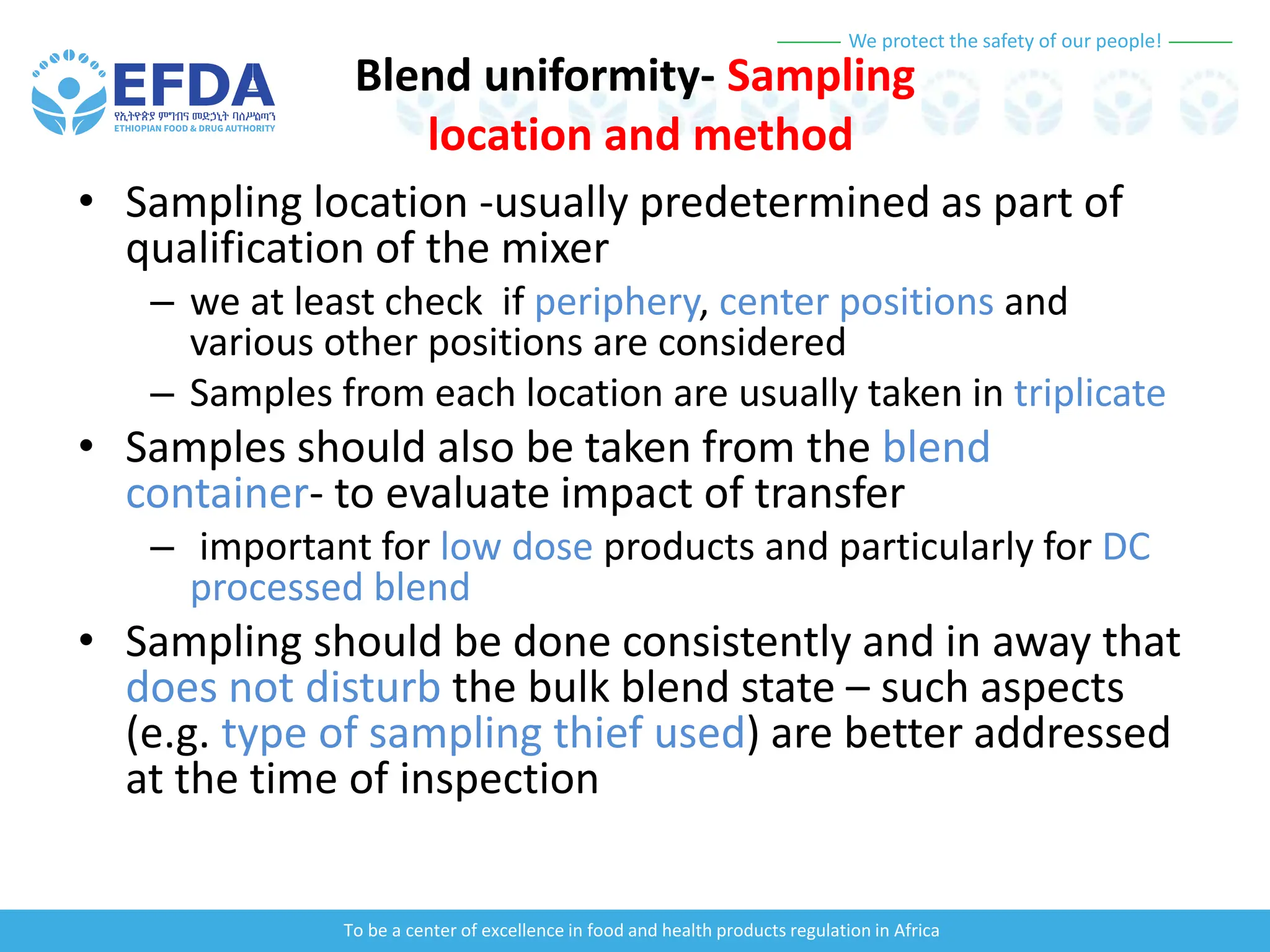
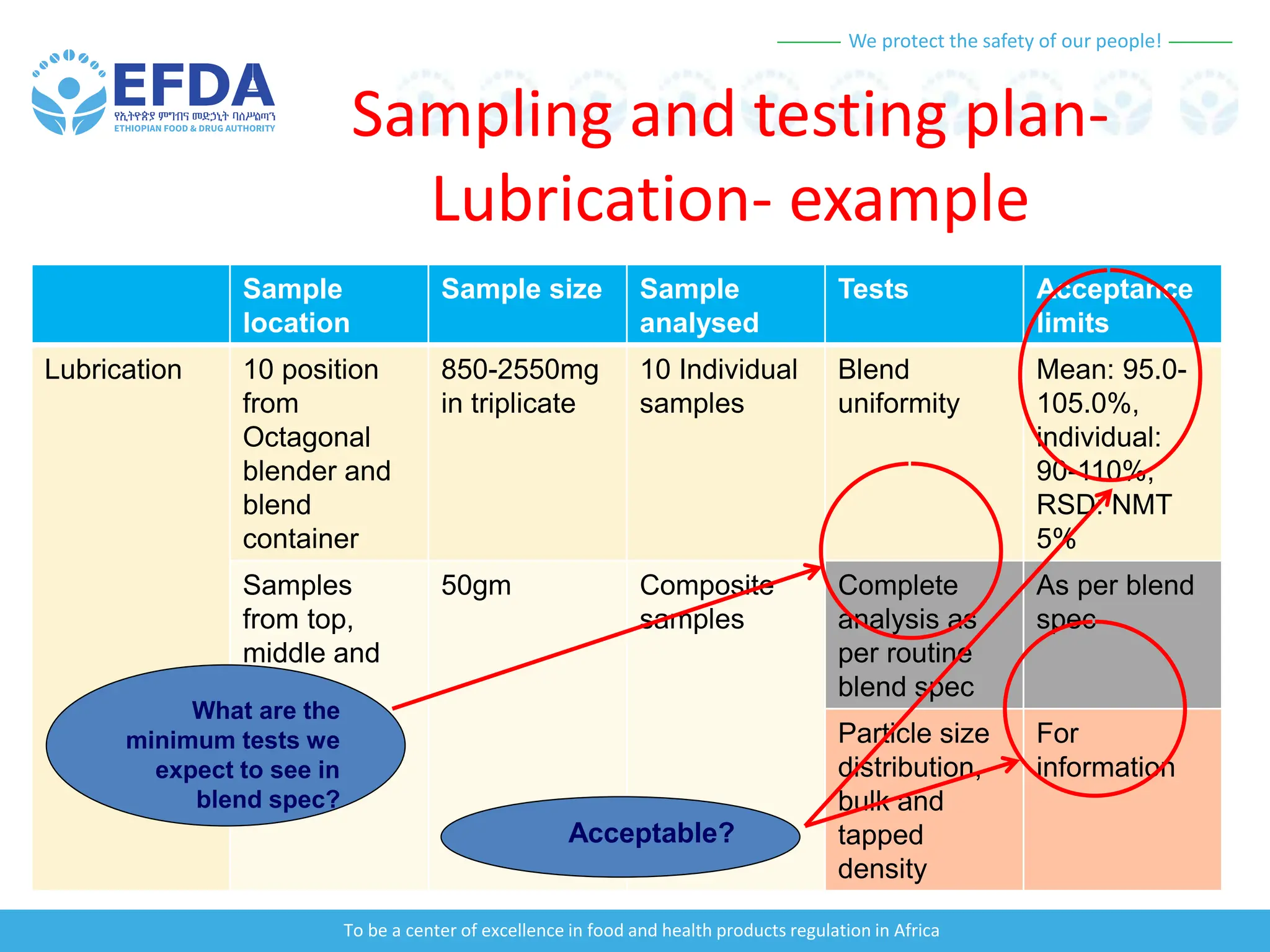
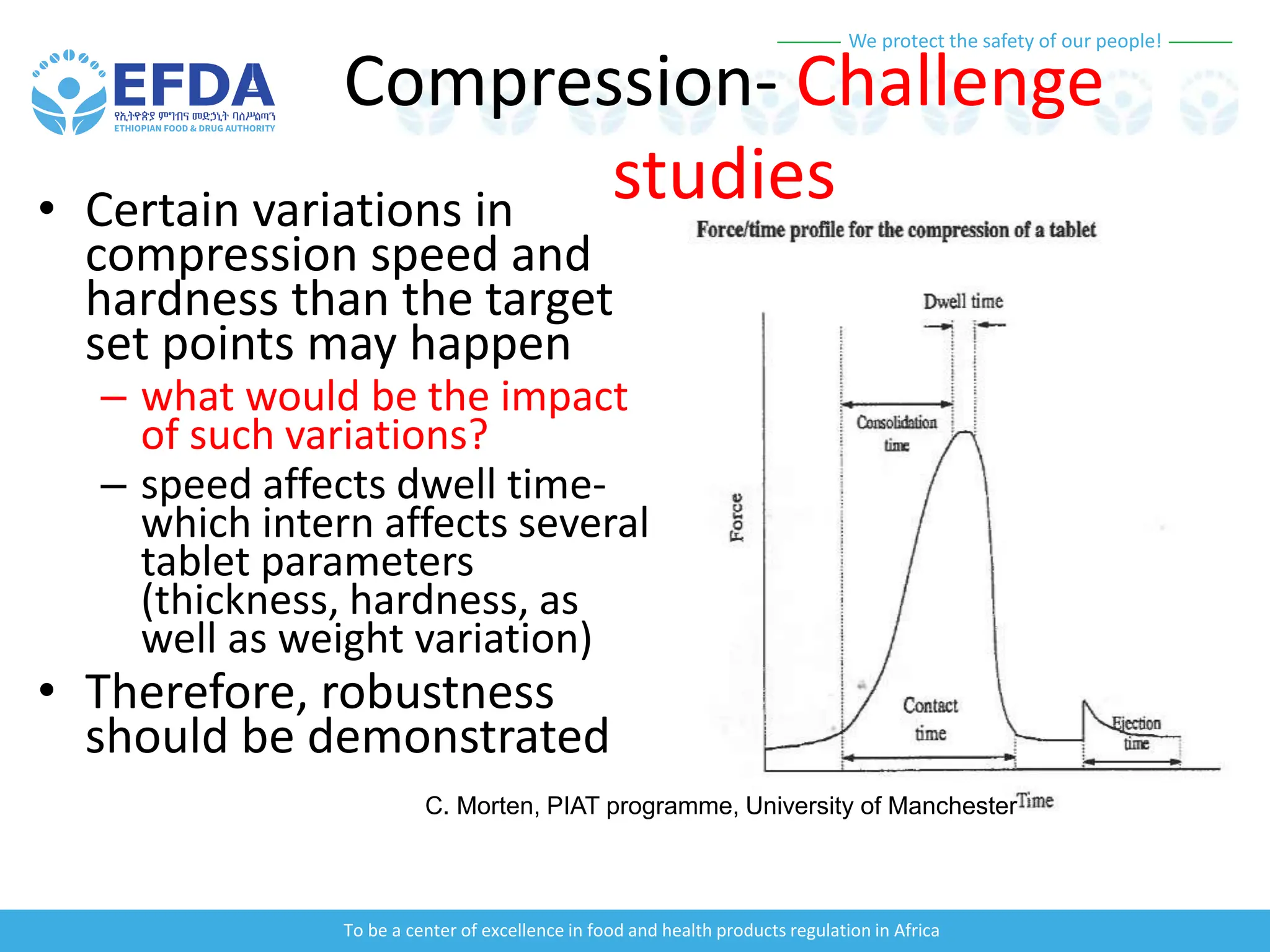
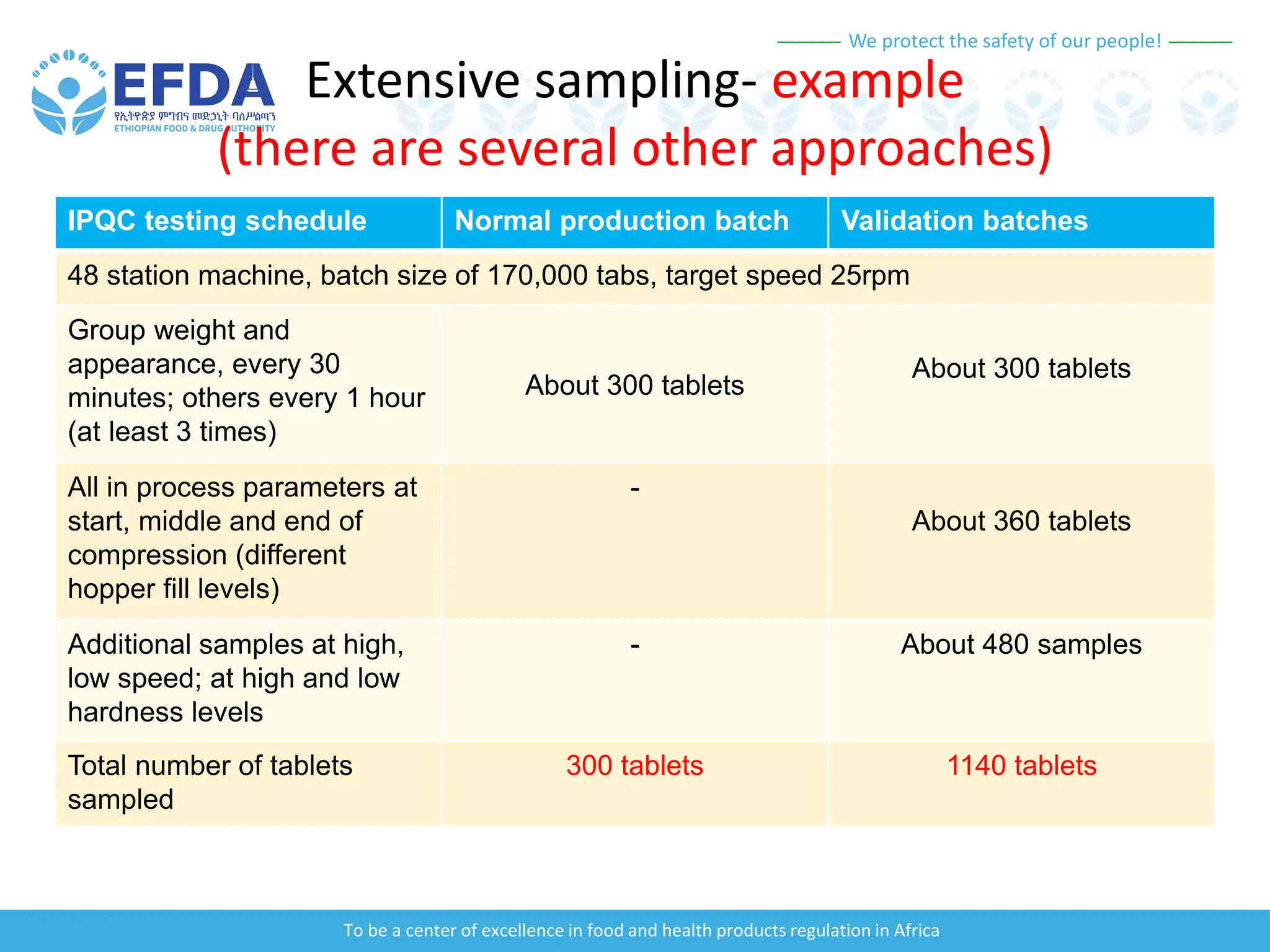
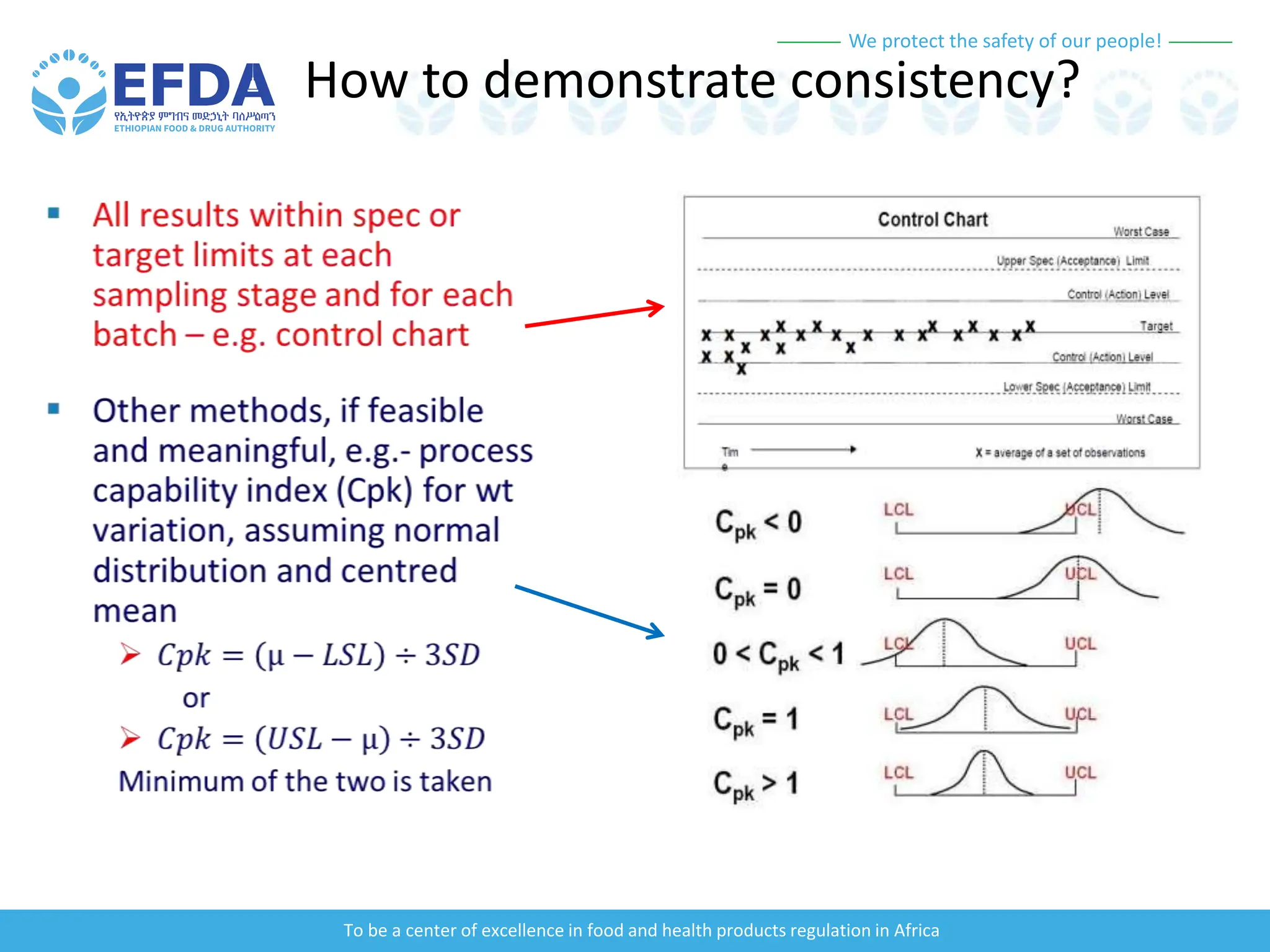
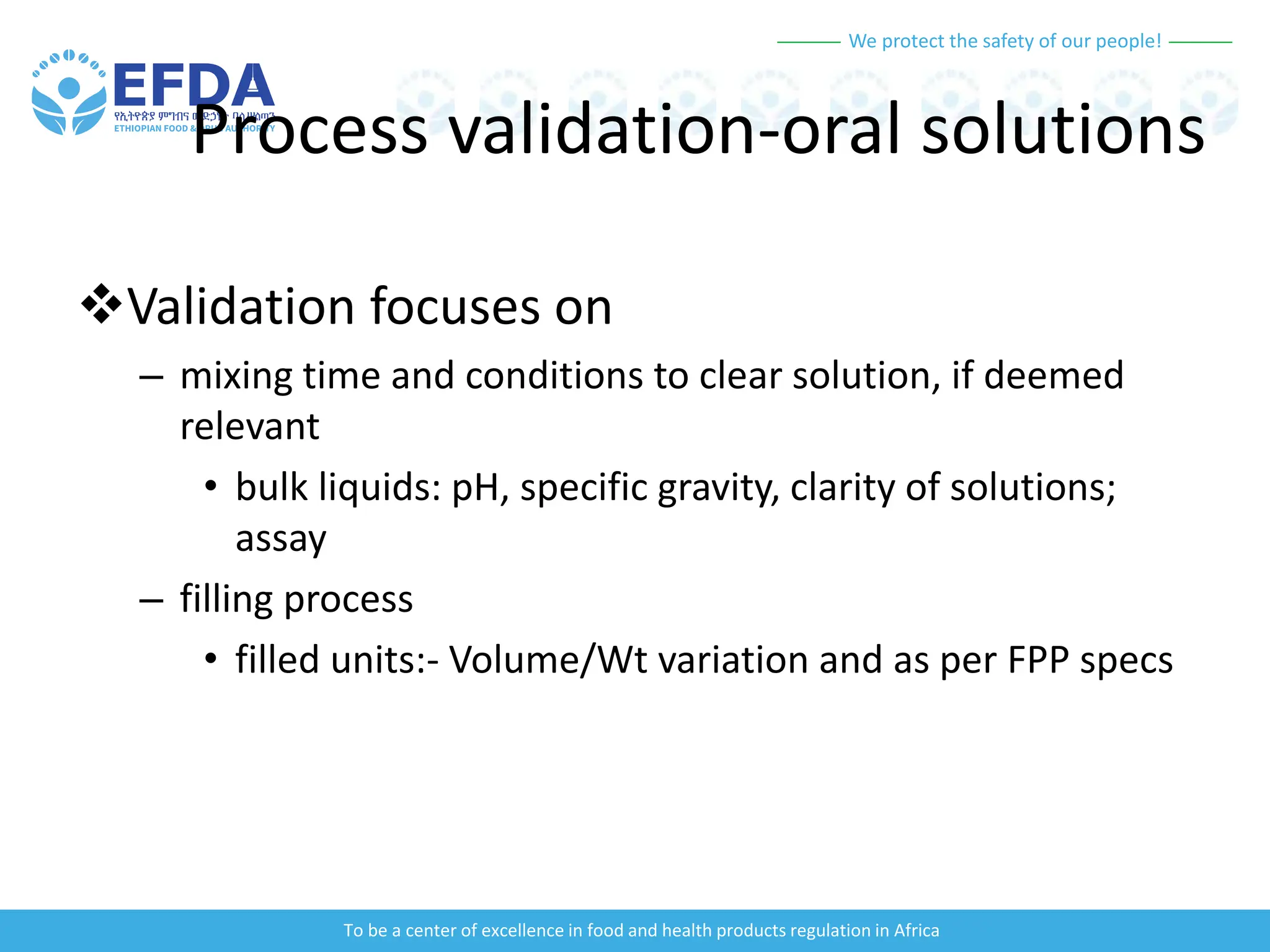
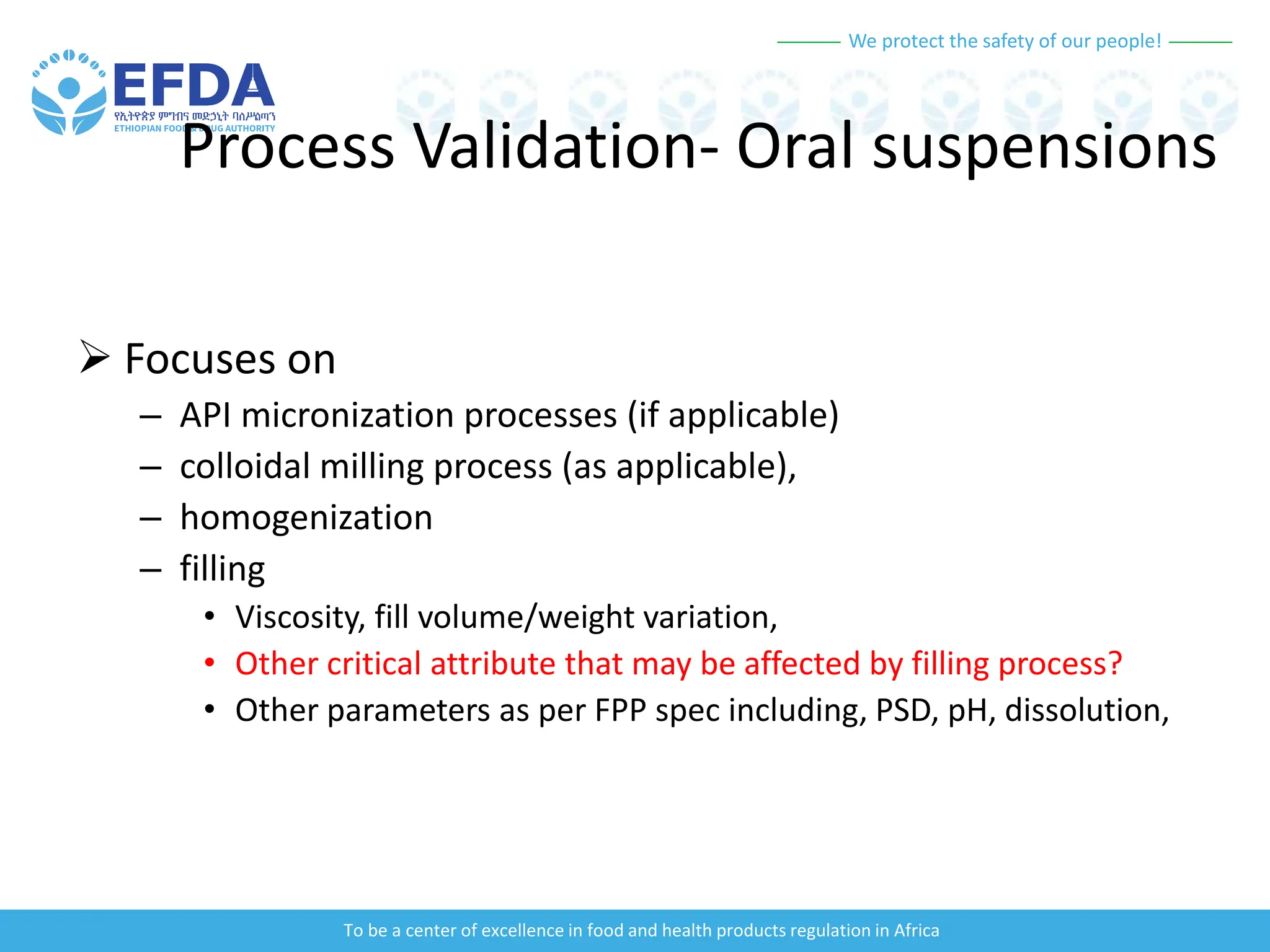
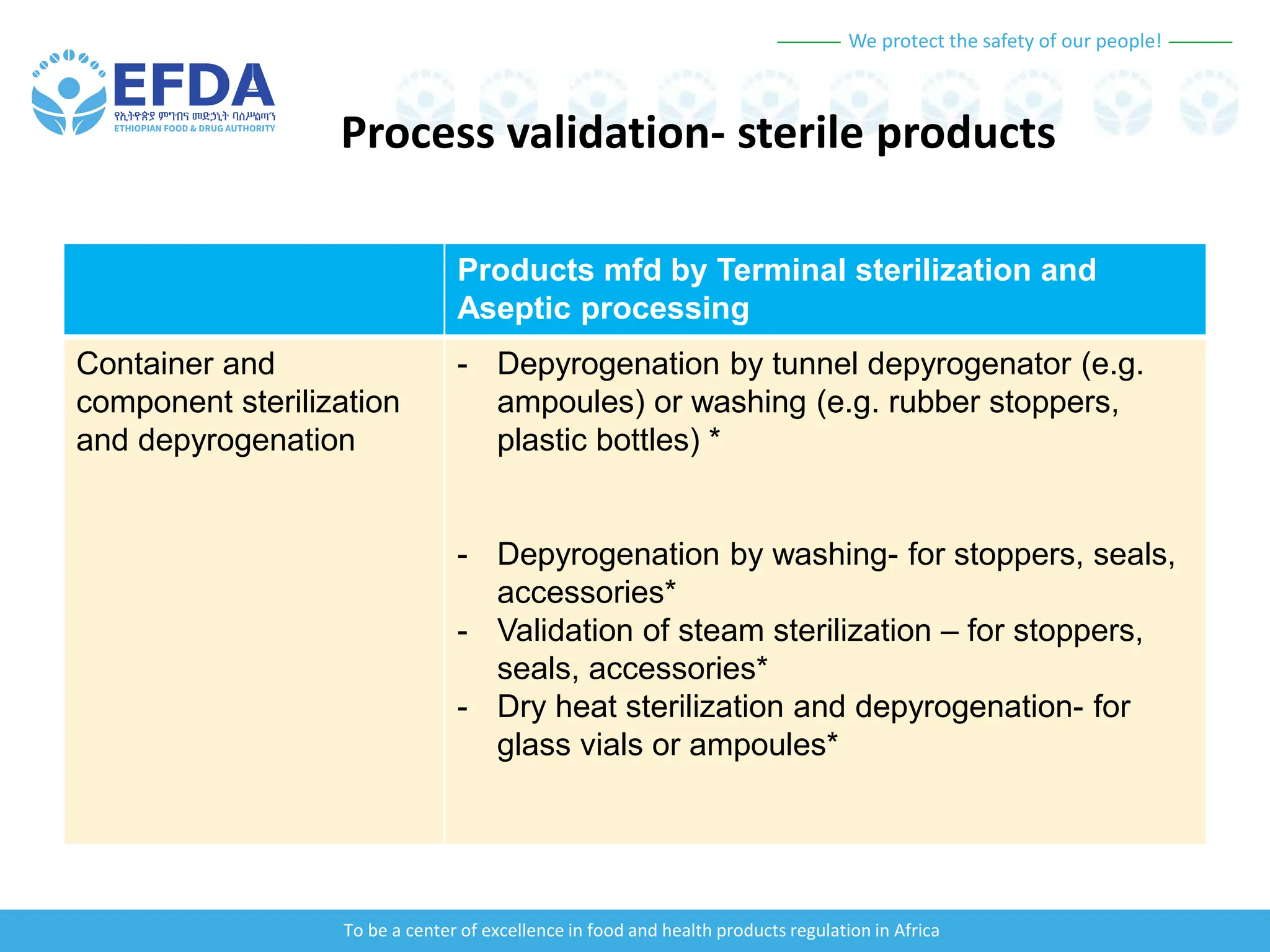
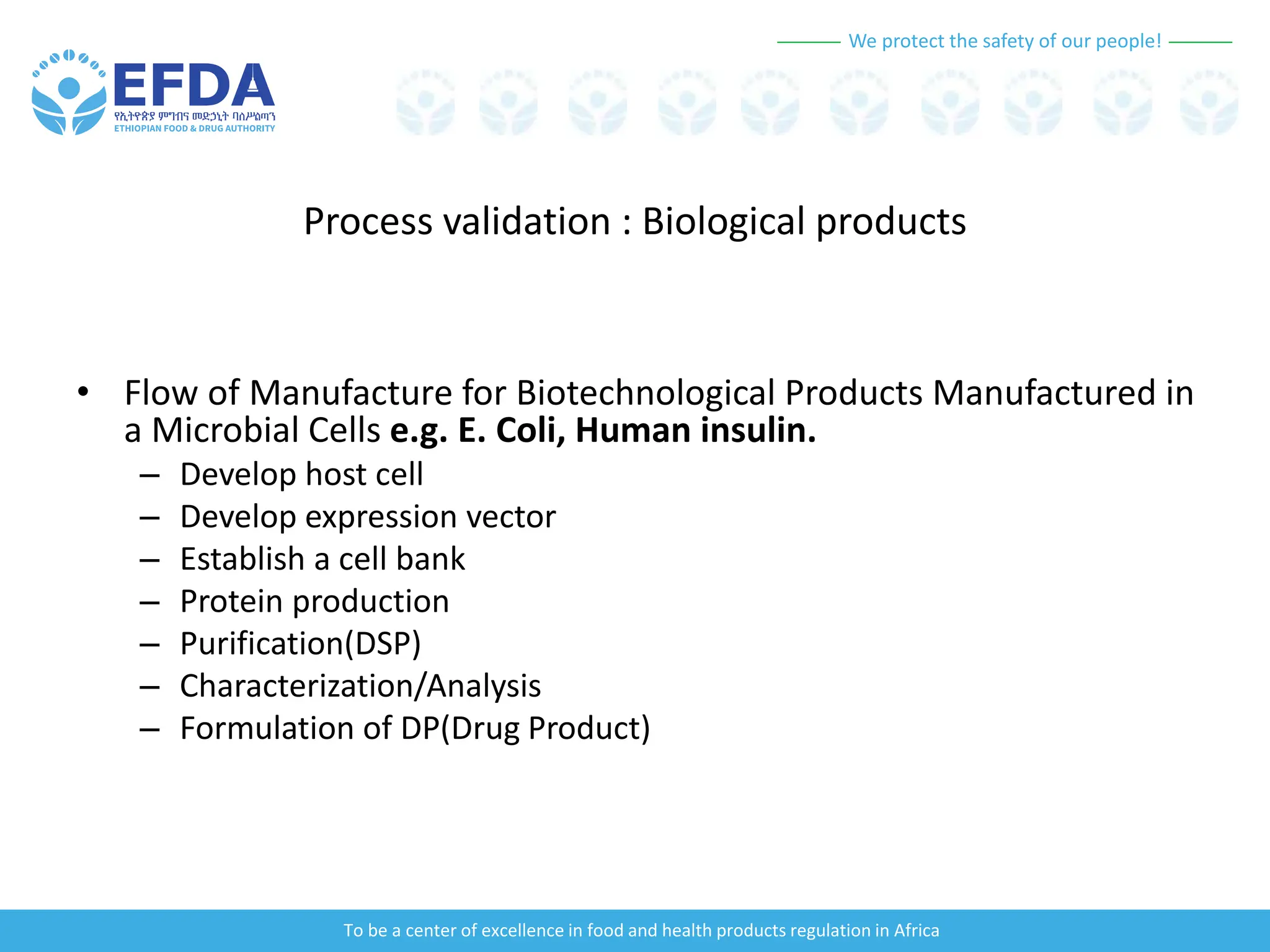
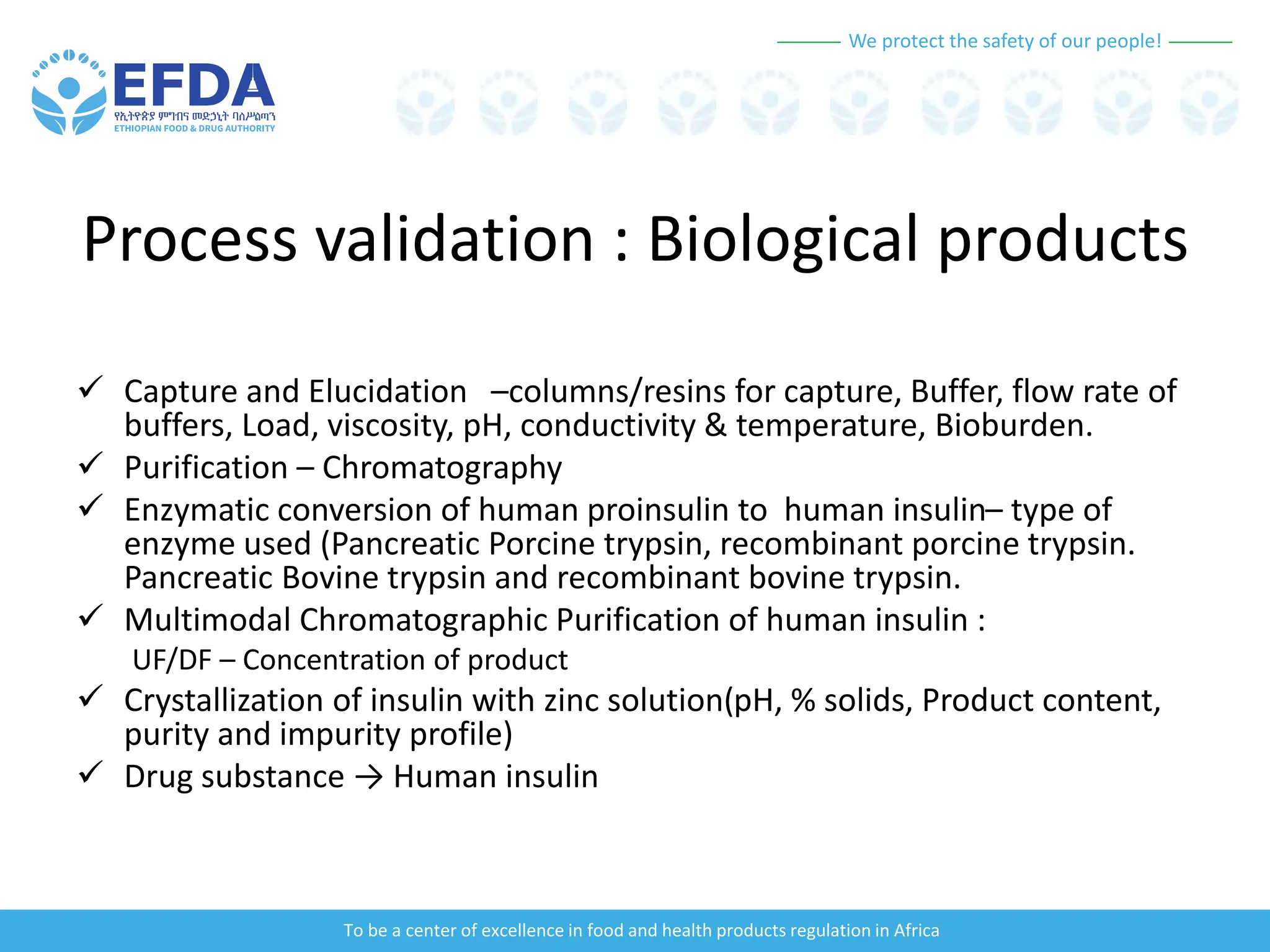
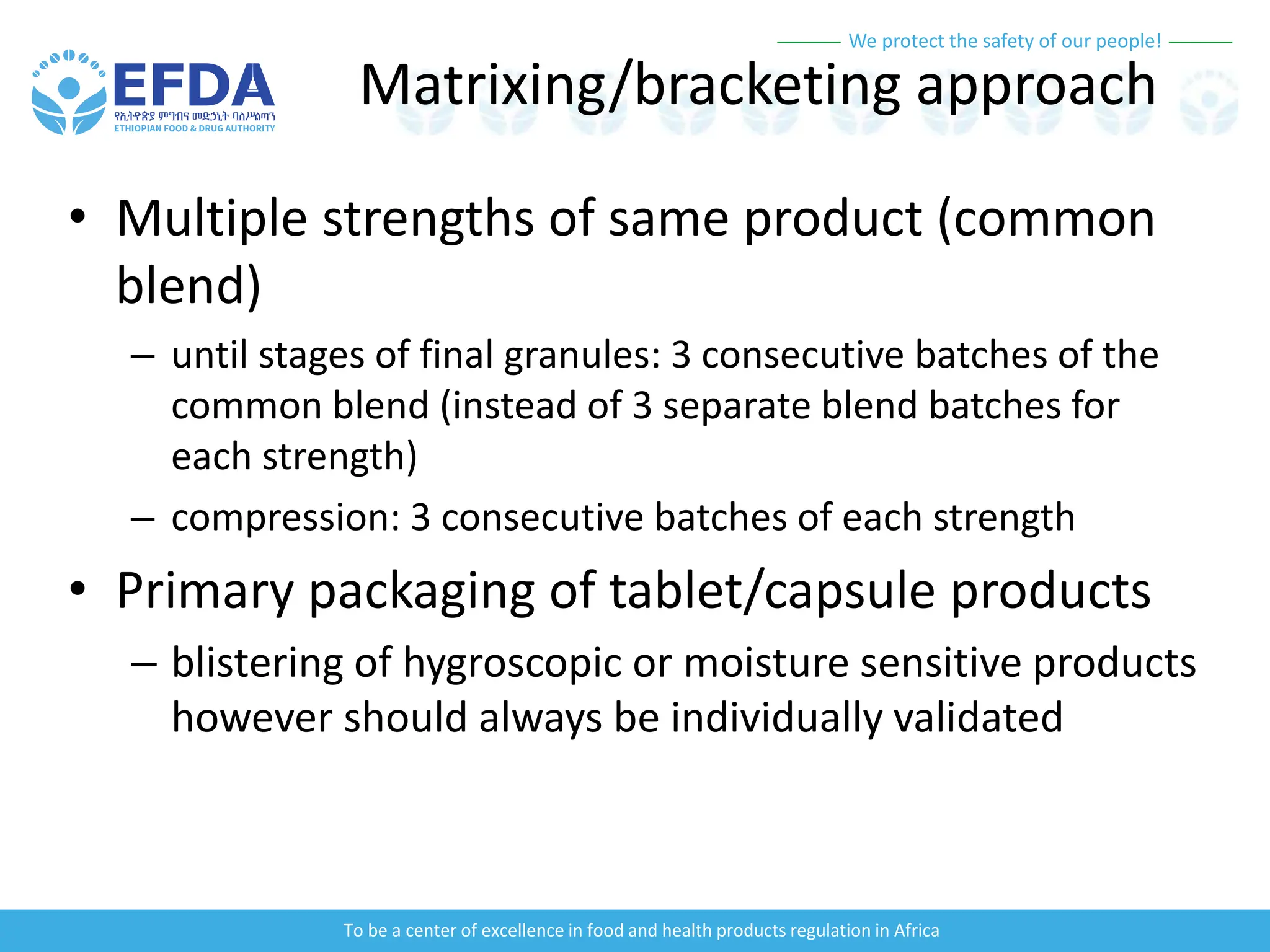
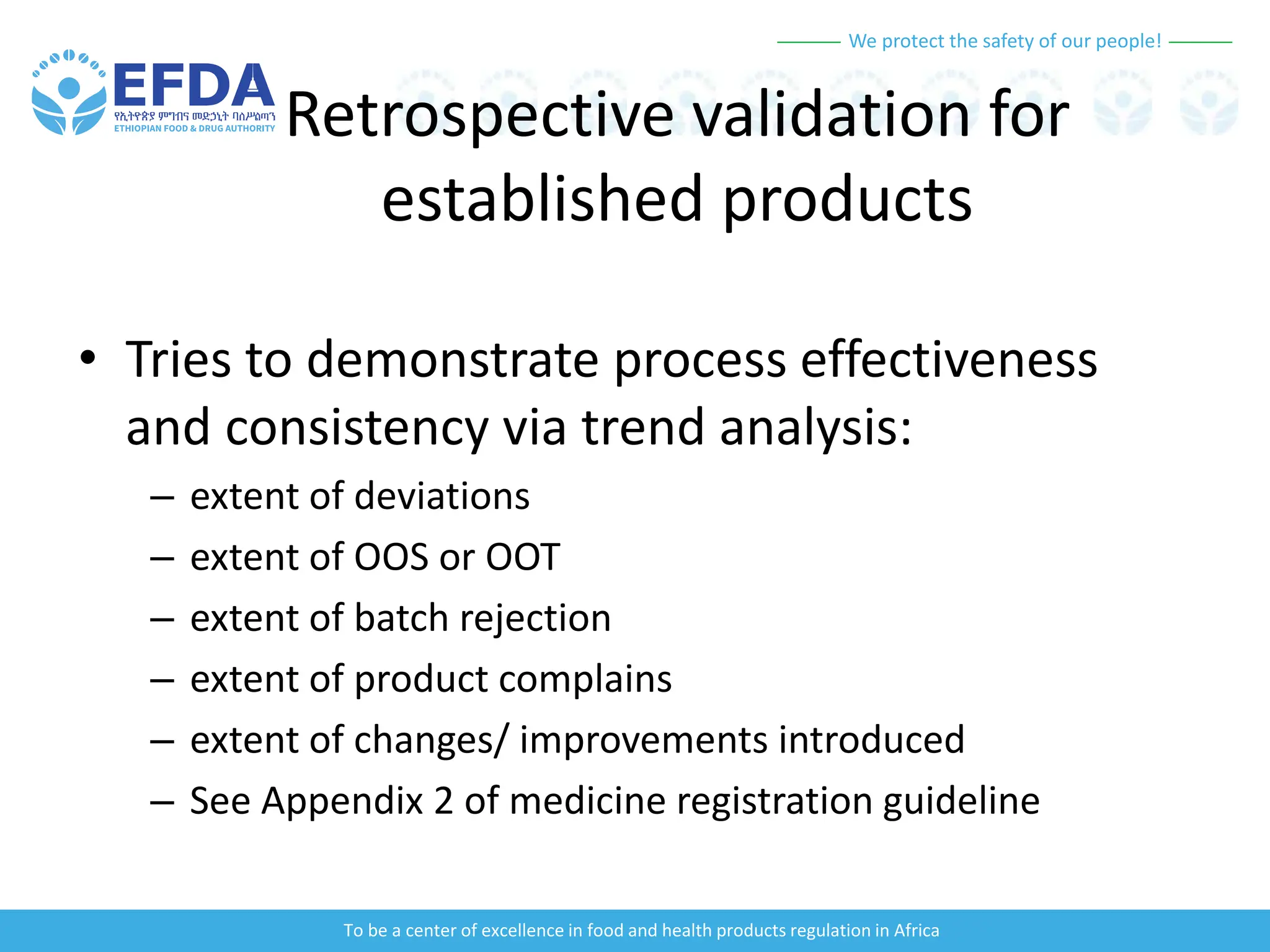
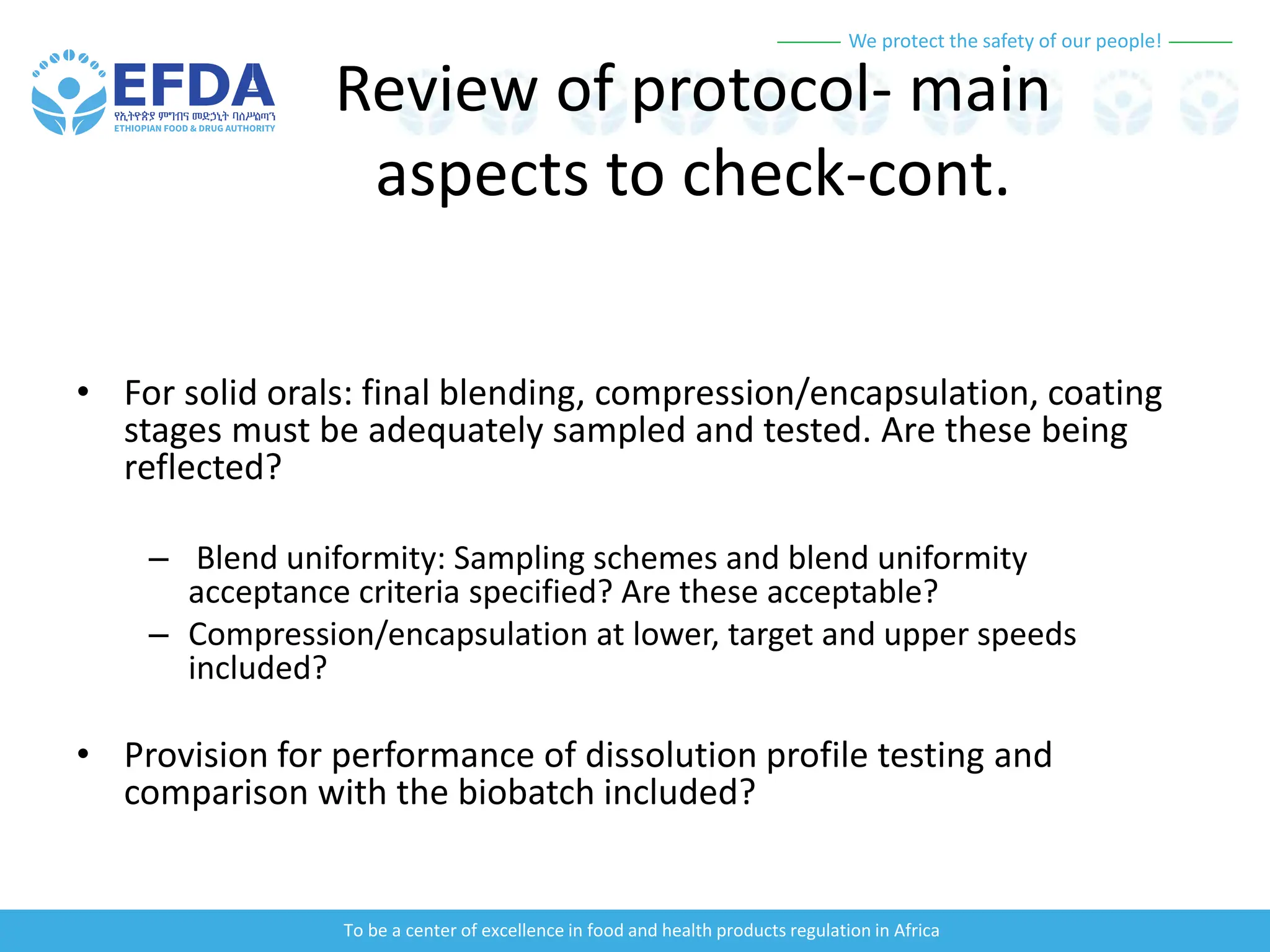
The document outlines the principles and processes of validation in food and health product regulation, emphasizing the importance of process validation for ensuring product quality. It details the definitions, objectives, and methodologies involved in traditional and continuous process validation approaches, including risk assessment and monitoring techniques for various stages of manufacturing. Additionally, the document provides insights into the critical quality attributes and validation schemes necessary for successful product development and market release.