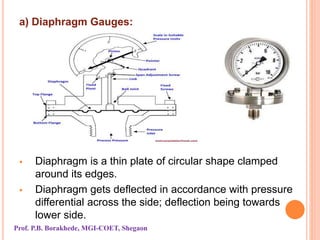
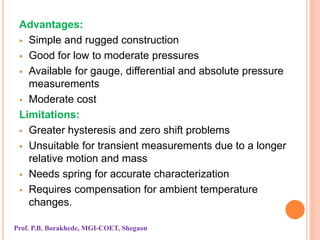
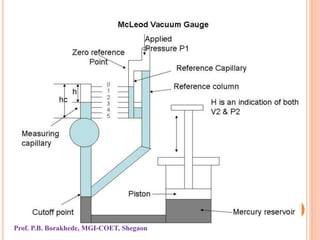
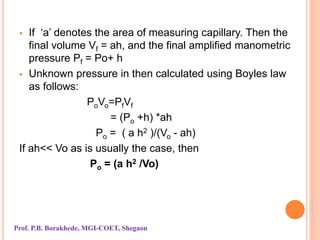
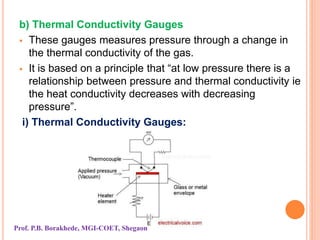
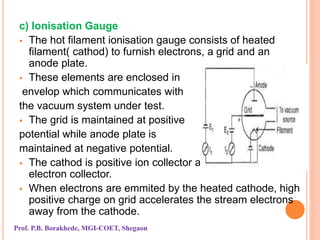
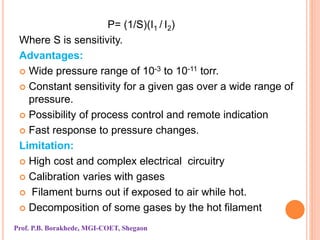
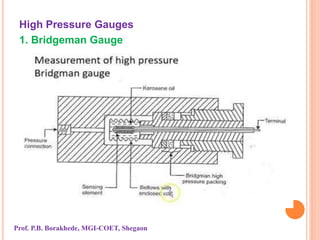
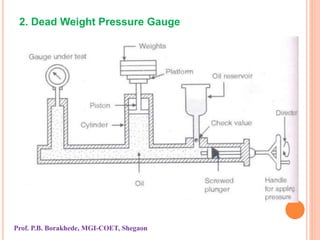
The document discusses various methods and devices for pressure measurement, which is crucial in industry and laboratories. It classifies pressure gauges into mechanical, low pressure, and high pressure categories, detailing subtypes such as diaphragm gauges, bellow gauges, and various low pressure measurement devices like McLeod and Pirani gauges. Each method and device has its own advantages and limitations, influencing its application and effectiveness in measuring pressure.