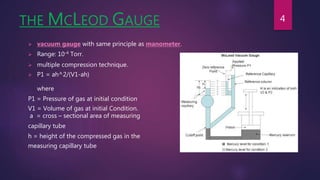
This document discusses various methods for measuring low pressures below 1 torr. It describes mechanical gauges like the McLeod gauge, thermal gauges like the Pirani gauge, and ionization gauges like hot cathode ionization gauges. The McLeod gauge uses multiple compression to indirectly measure pressure based on gas volume and height. The Pirani gauge measures changes in electrical resistance of a heated wire as pressure and gas conductivity changes. Ionization gauges produce ions from electron bombardment and measure pressure through ratios of collected currents. Absolute techniques like the Knudsen gauge and Alphatron gauge also are discussed which relate pressure to momentum transfer or ionization from radioactive sources.