The document discusses the stoichiometry of combustion reactions, focusing on both volumetric and gravimetric analysis, particularly in the context of fossil fuels like hydrocarbons. It outlines combustion equations for solid and gaseous fuels while providing examples of how to calculate the theoretical air requirement for complete combustion. Various oxidizers used in combustion processes are also mentioned, along with their implications in chemical reactions.




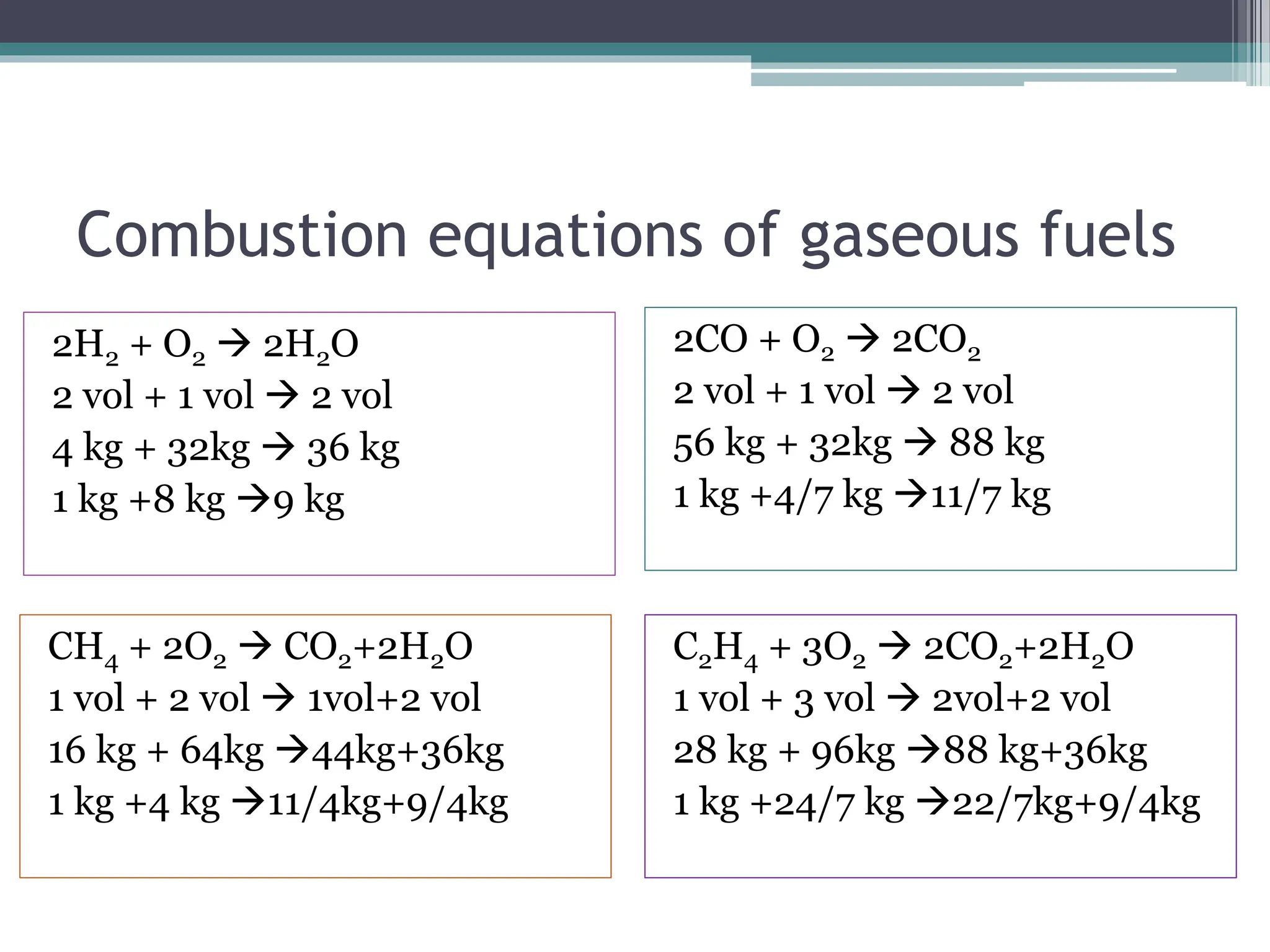
![Theoretical air requirement
= 100/23 [(8/3C + 8H2 + S)-O2 ] kg
= 100/21 [(0.5CO+ 0.5H2 +2CH4 + 3C2H4)-O2 ]m3](https://image.slidesharecdn.com/stoichiometryofcombustion-240129013111-24d40102/75/Stoichiometry-of-combustion-of-fuels-pptx-6-2048.jpg)


