This document provides an introduction to thermogravimetric analysis (TGA), differential thermal analysis (DTA), and differential scanning calorimetry (DSC). It discusses key concepts like how TGA measures weight changes as a function of temperature, allowing one to determine characteristics of thermal decomposition reactions. Examples are given of TGA curves for silver nitrate and cupric nitrate hemipentahydrate to illustrate how different materials may experience one or multiple steps of weight loss at different temperatures. The document also briefly introduces DTA as measuring the temperature difference between a sample and reference as they are heated.




![{
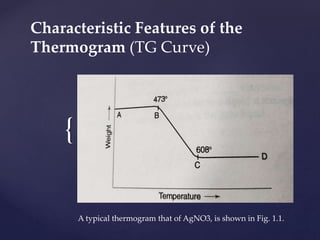
It is possible that there can be more than one step in
which weight losses can occur. The thermogram (Fig.
1.2) of cupric nitrate hemipentahydrate [Cu(NO3)2
2.5H2O] illustrates this point.
Fig. 1.2 TGA of cupric nitrate hemipentahydrate](https://image.slidesharecdn.com/presentationtg1-200812074741/85/Presentation-thermogravimetry-9-320.jpg)


