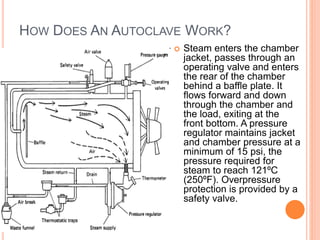
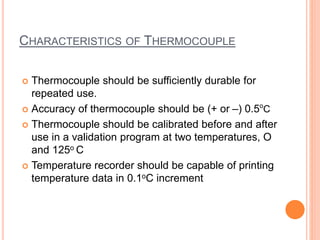
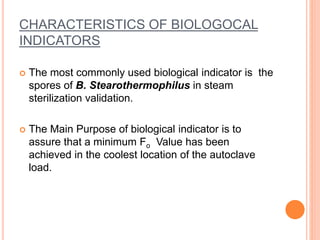
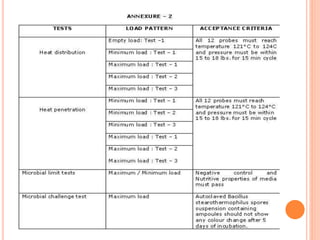
Steam sterilization uses high-pressure steam to kill microorganisms. It is the most effective sterilization method when heat and moisture do not damage products. An autoclave uses steam to heat items inside a pressurized chamber, reaching temperatures over 121°C. Validation of a sterilization process involves qualification of the autoclave design, installation, operation, and performance through studies measuring temperature distribution, heat penetration, and using biological indicators to confirm a sterilization log reduction factor is achieved.