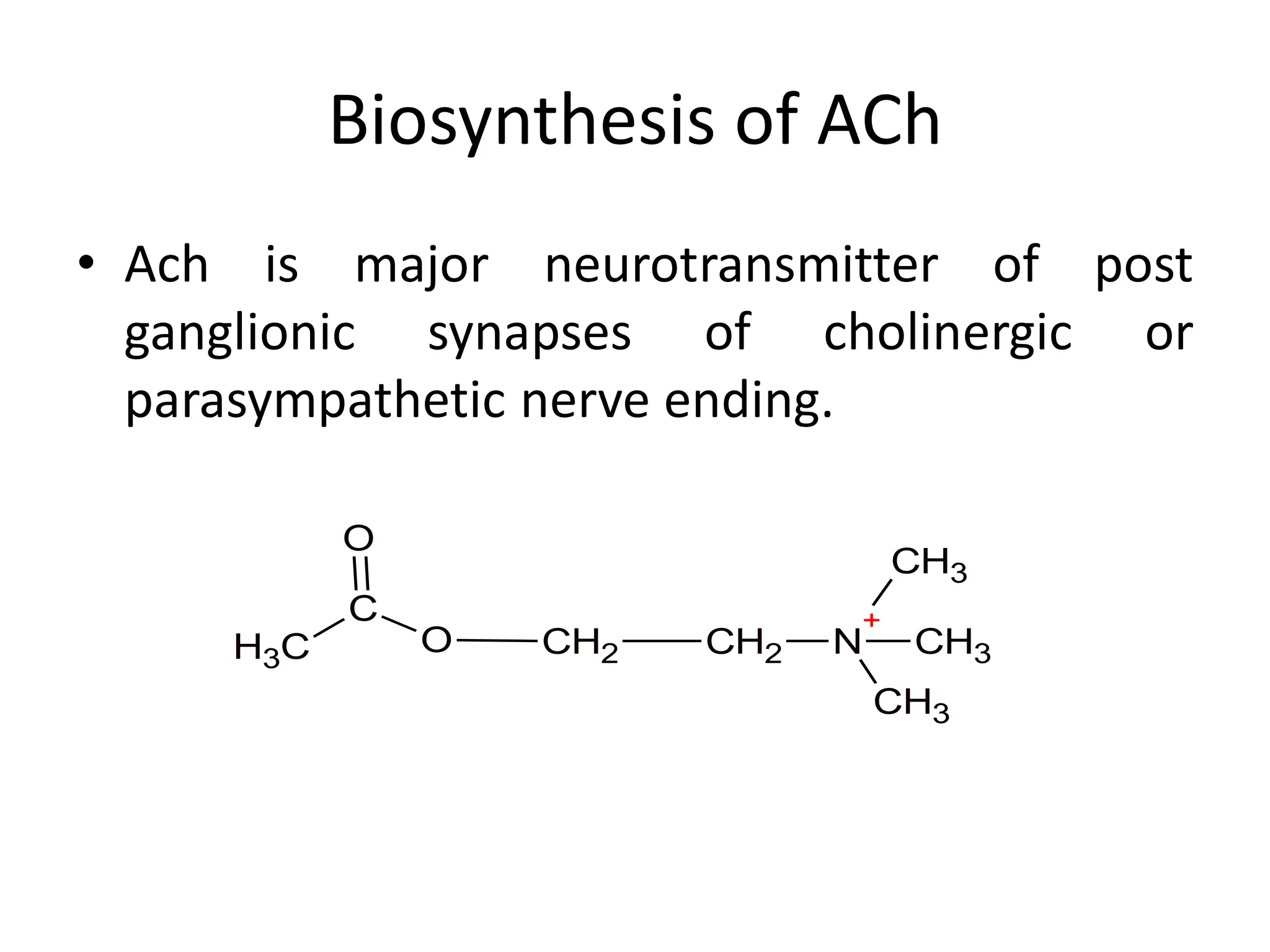
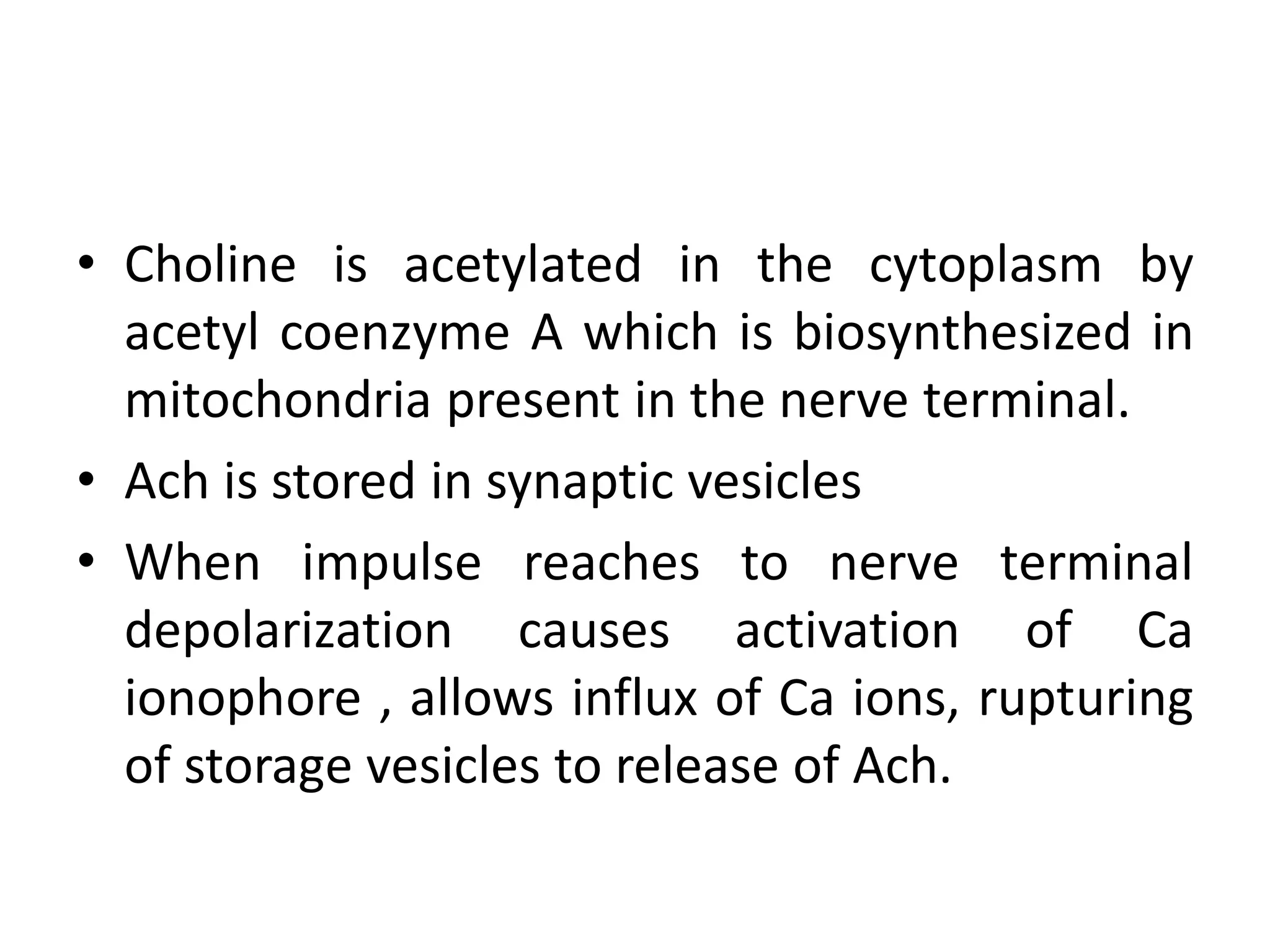
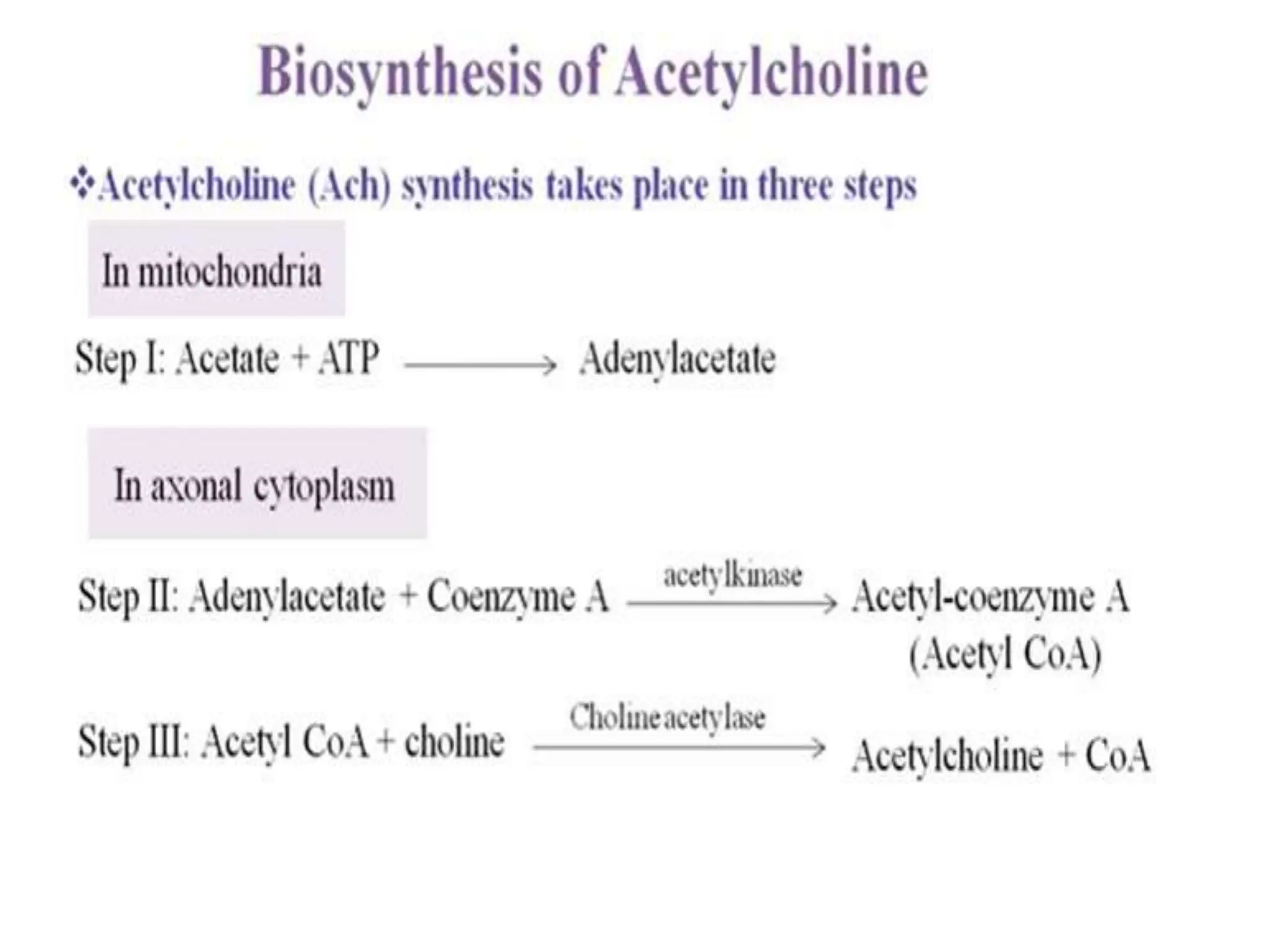
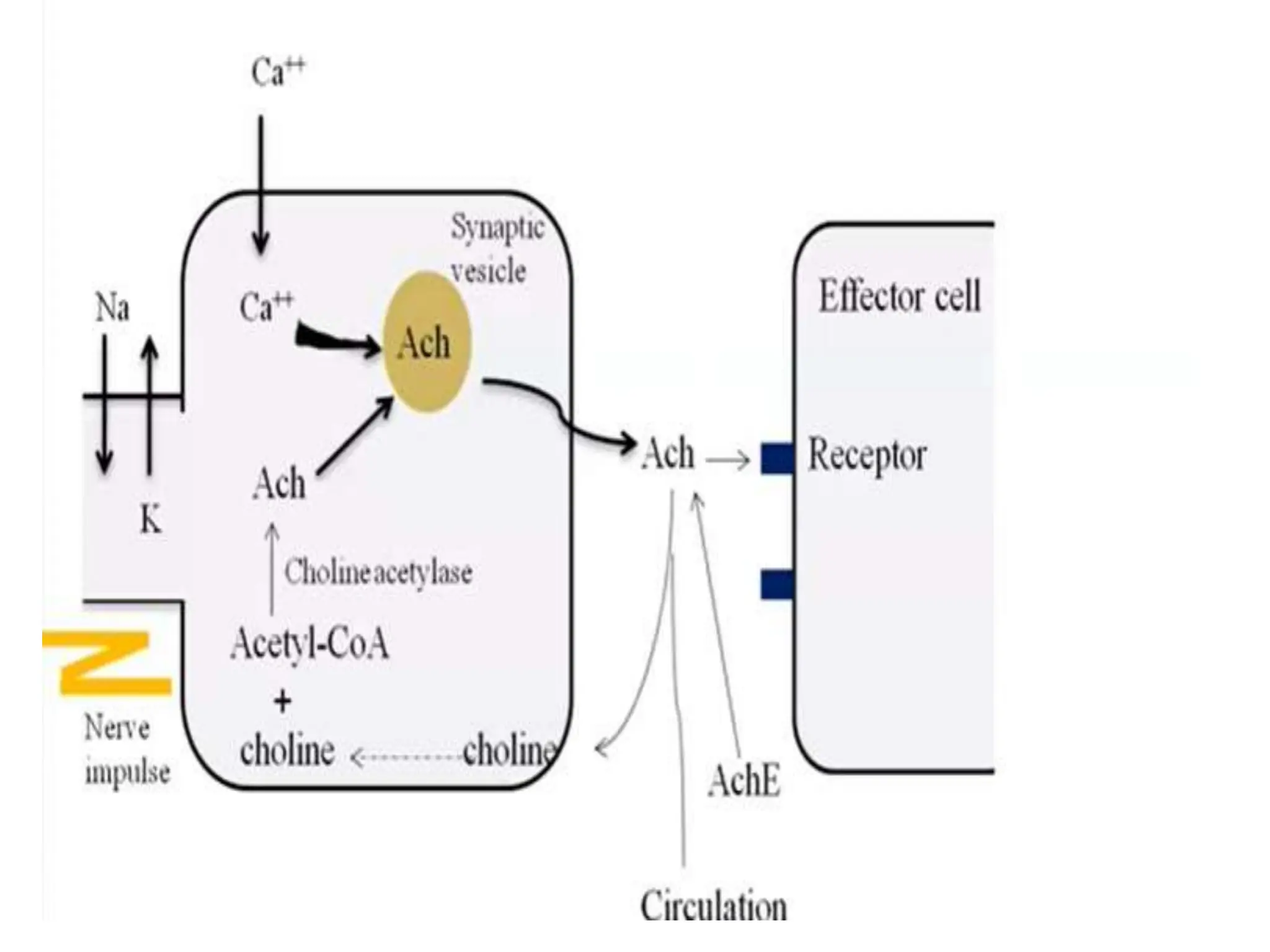
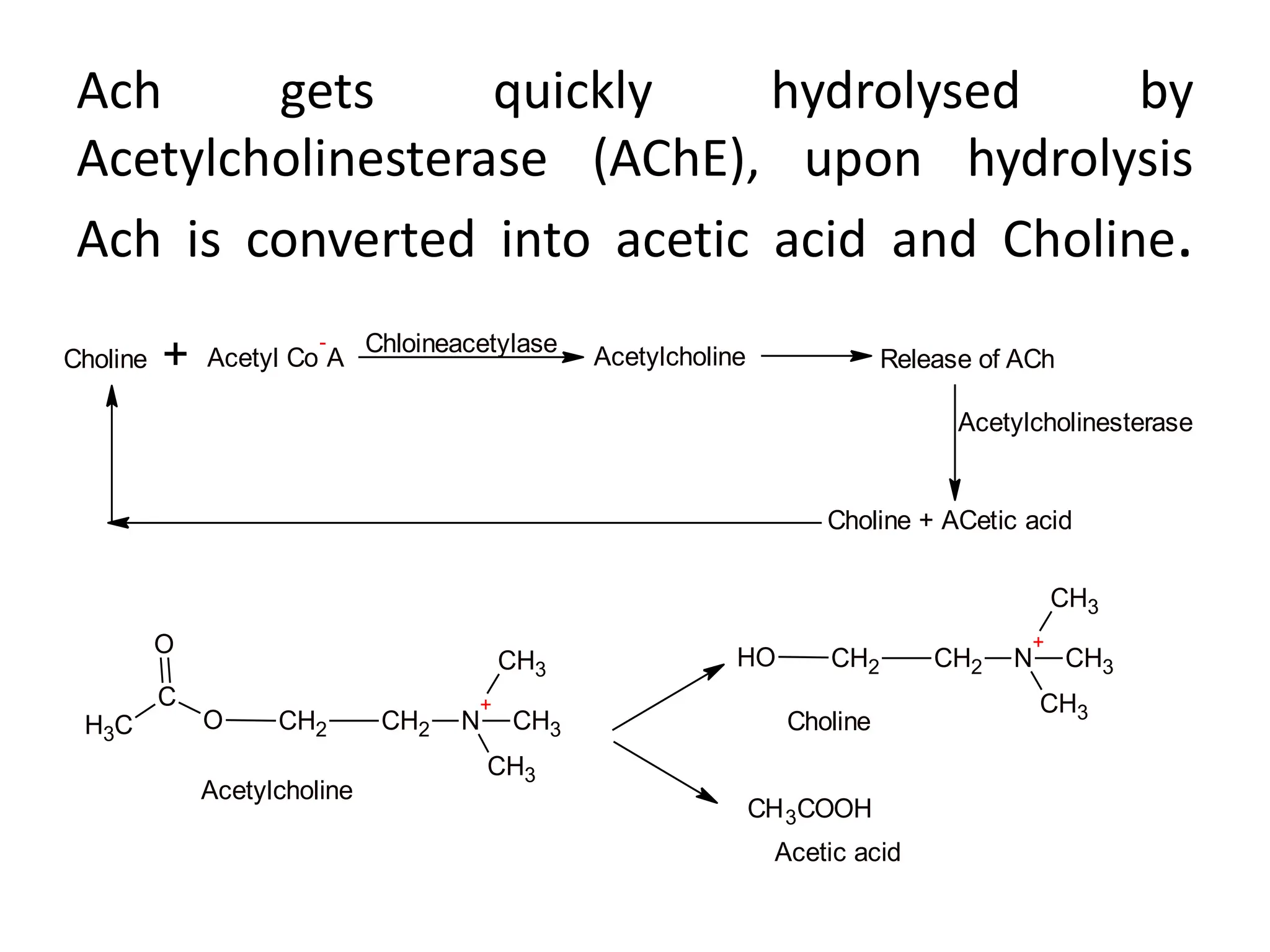
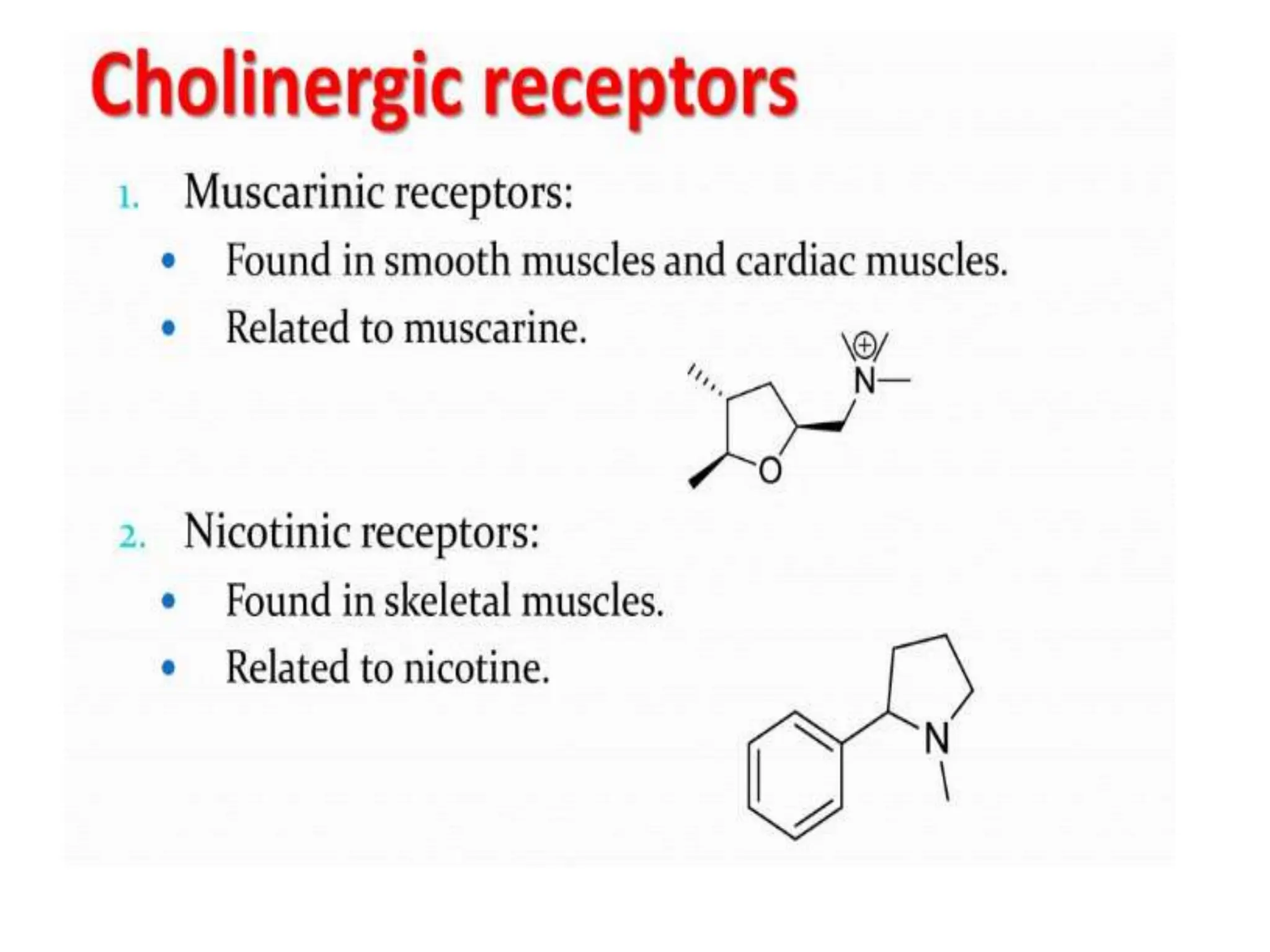
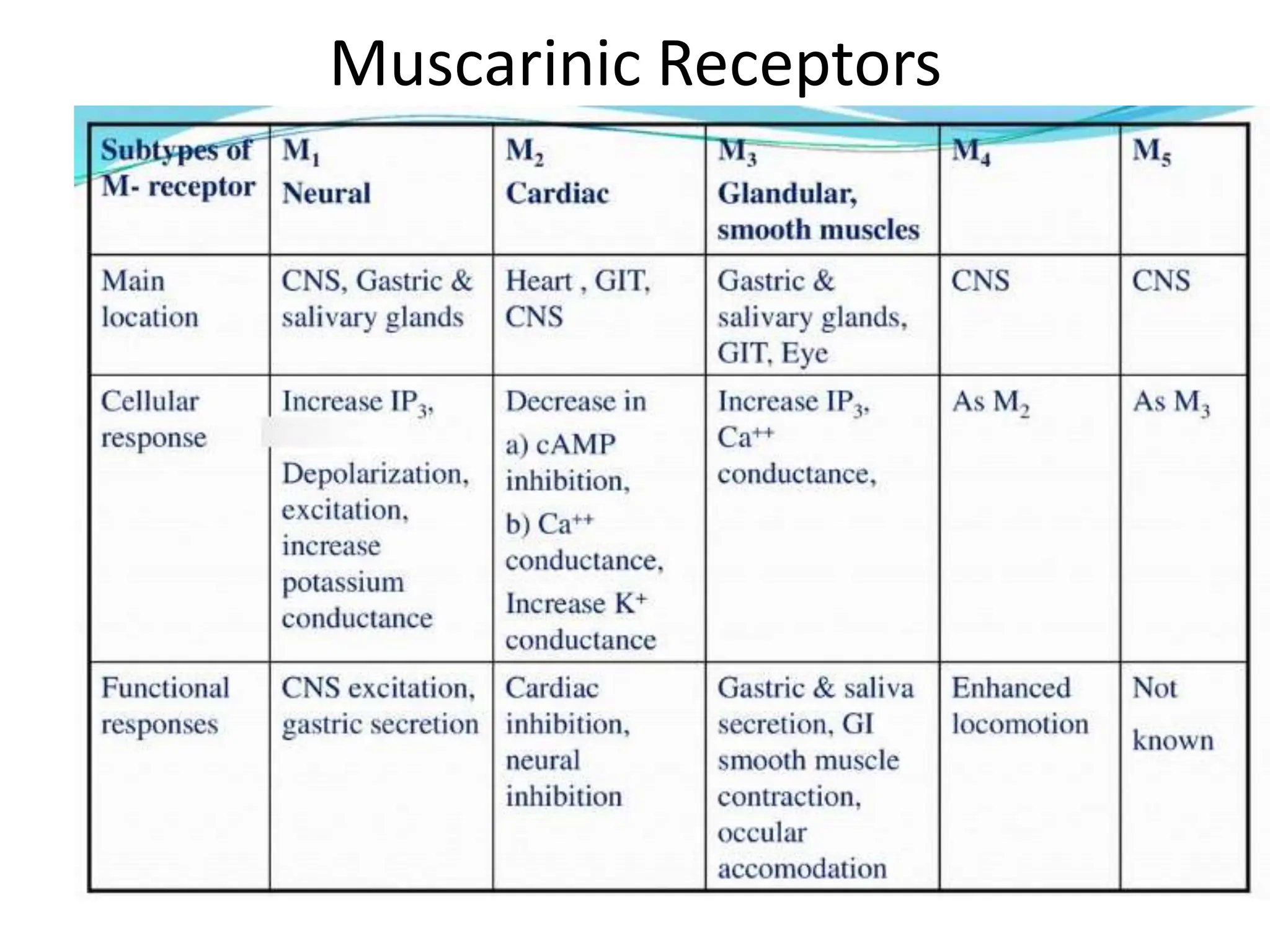
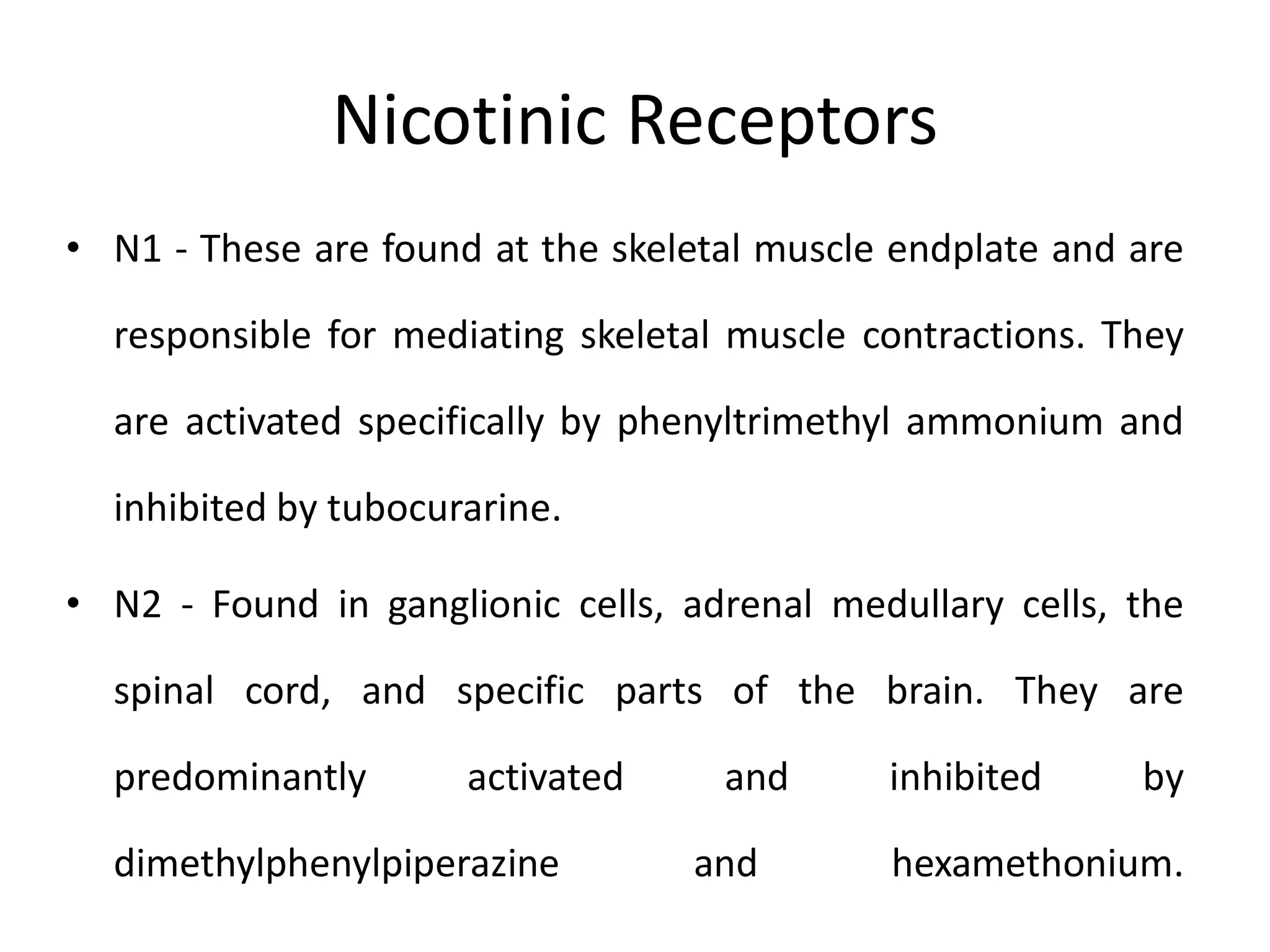
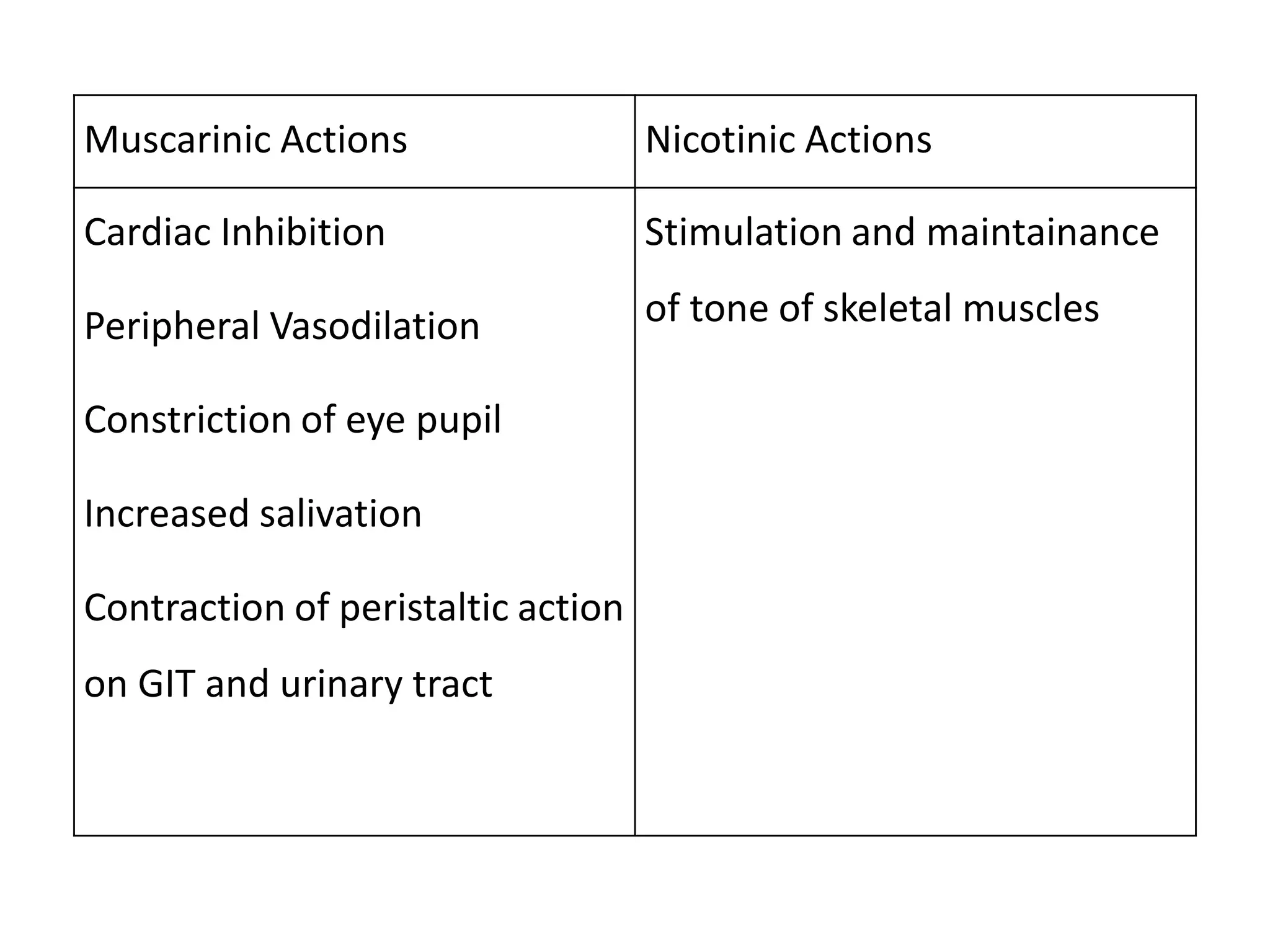
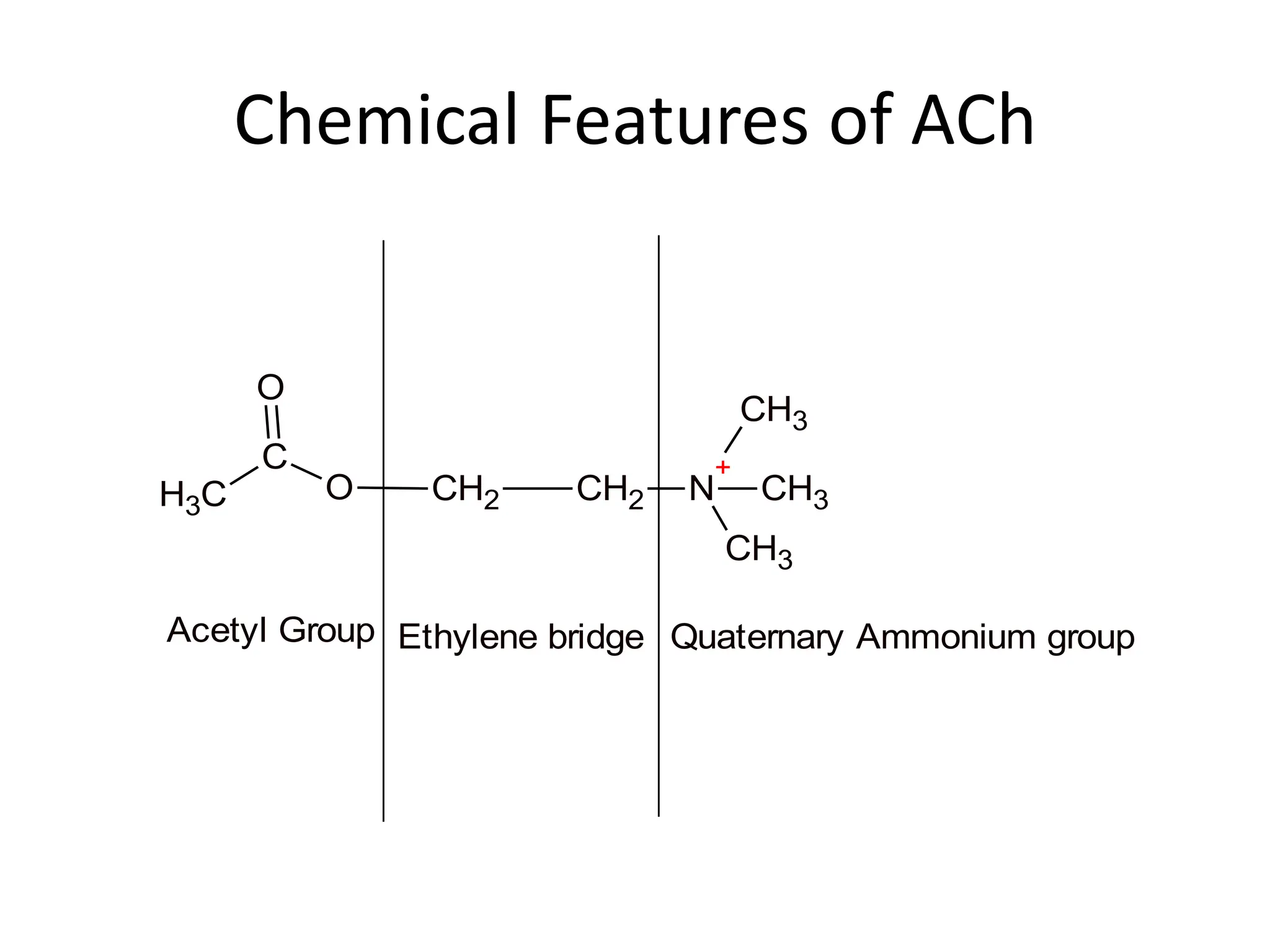
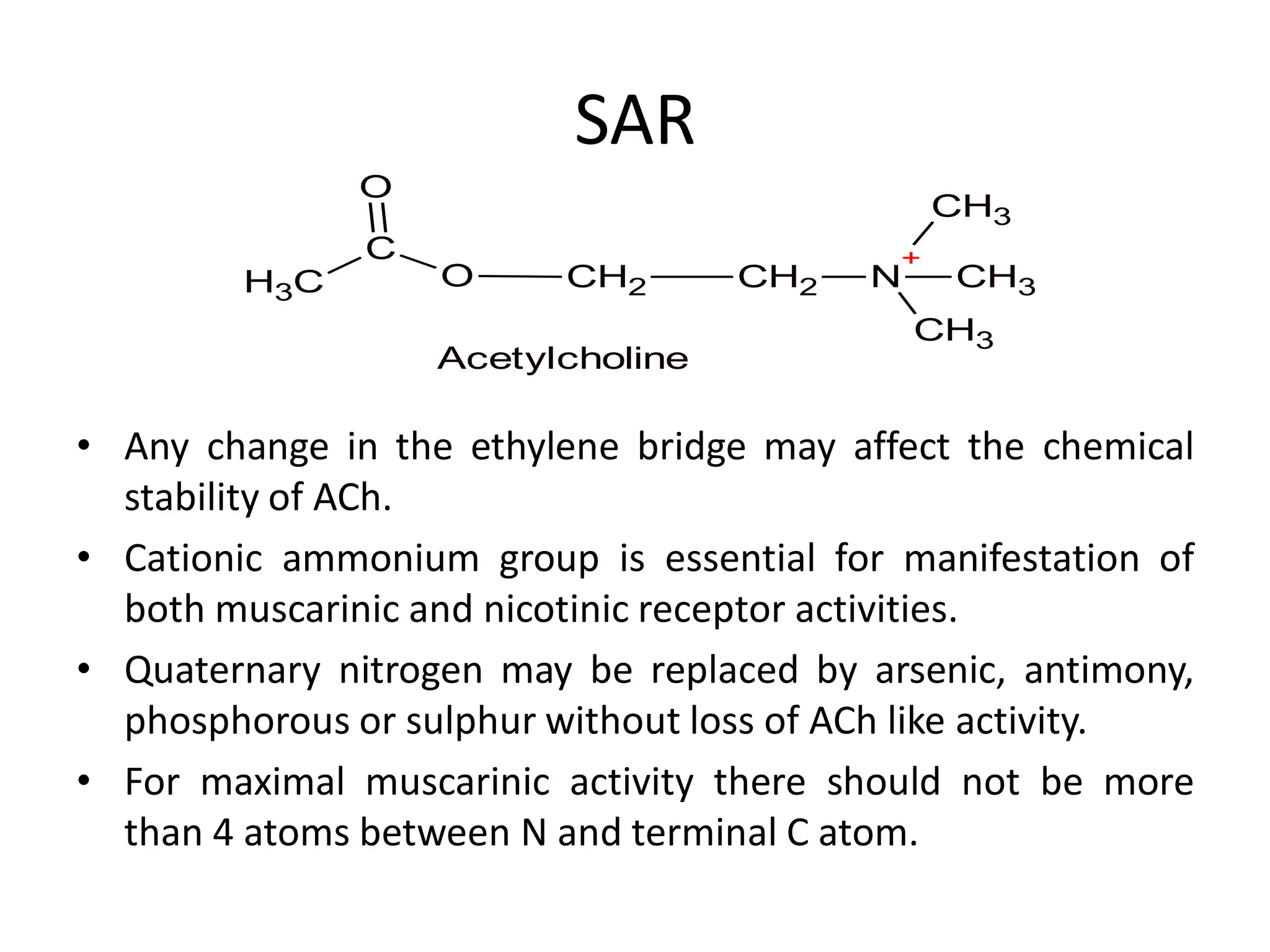
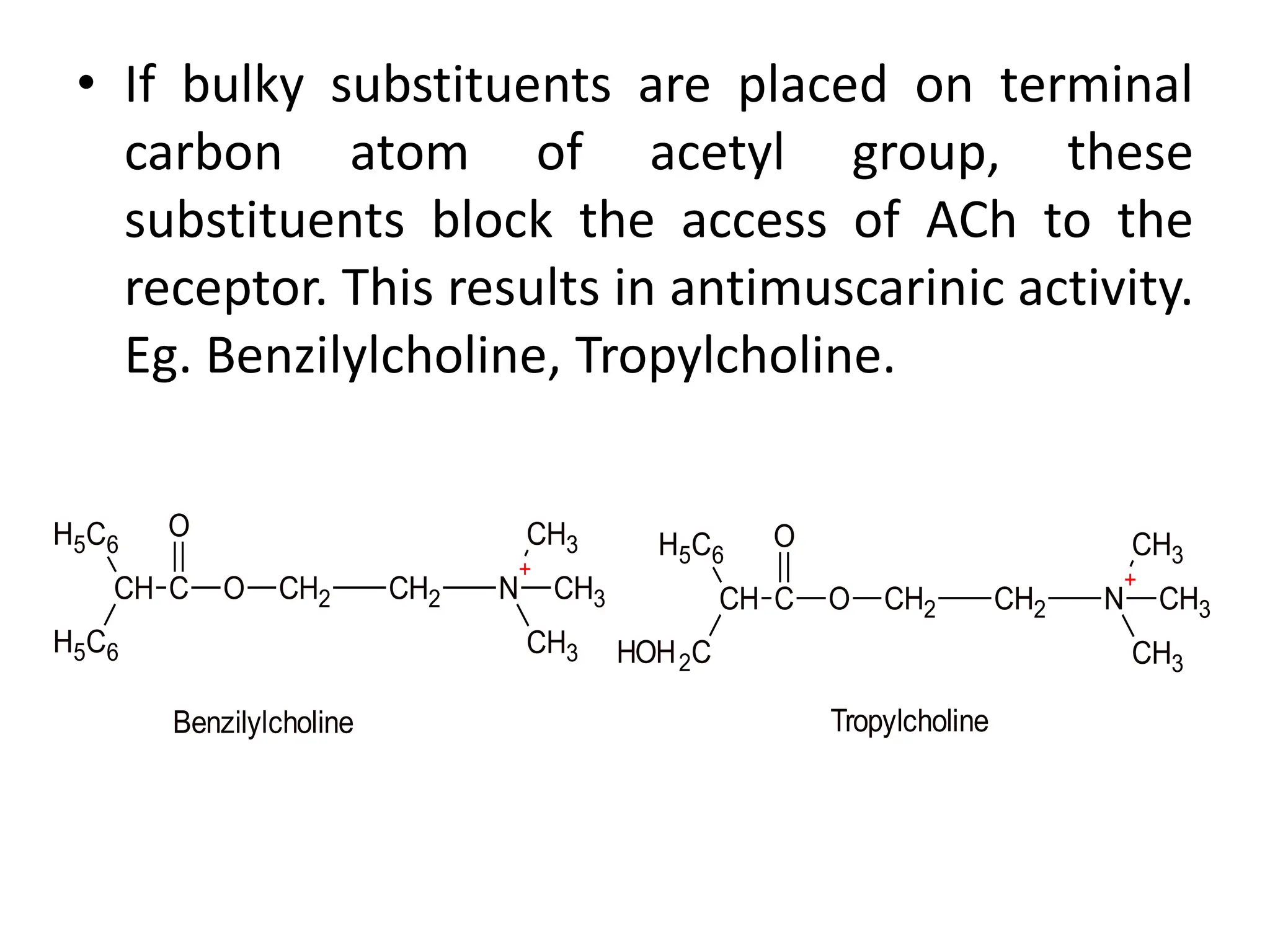
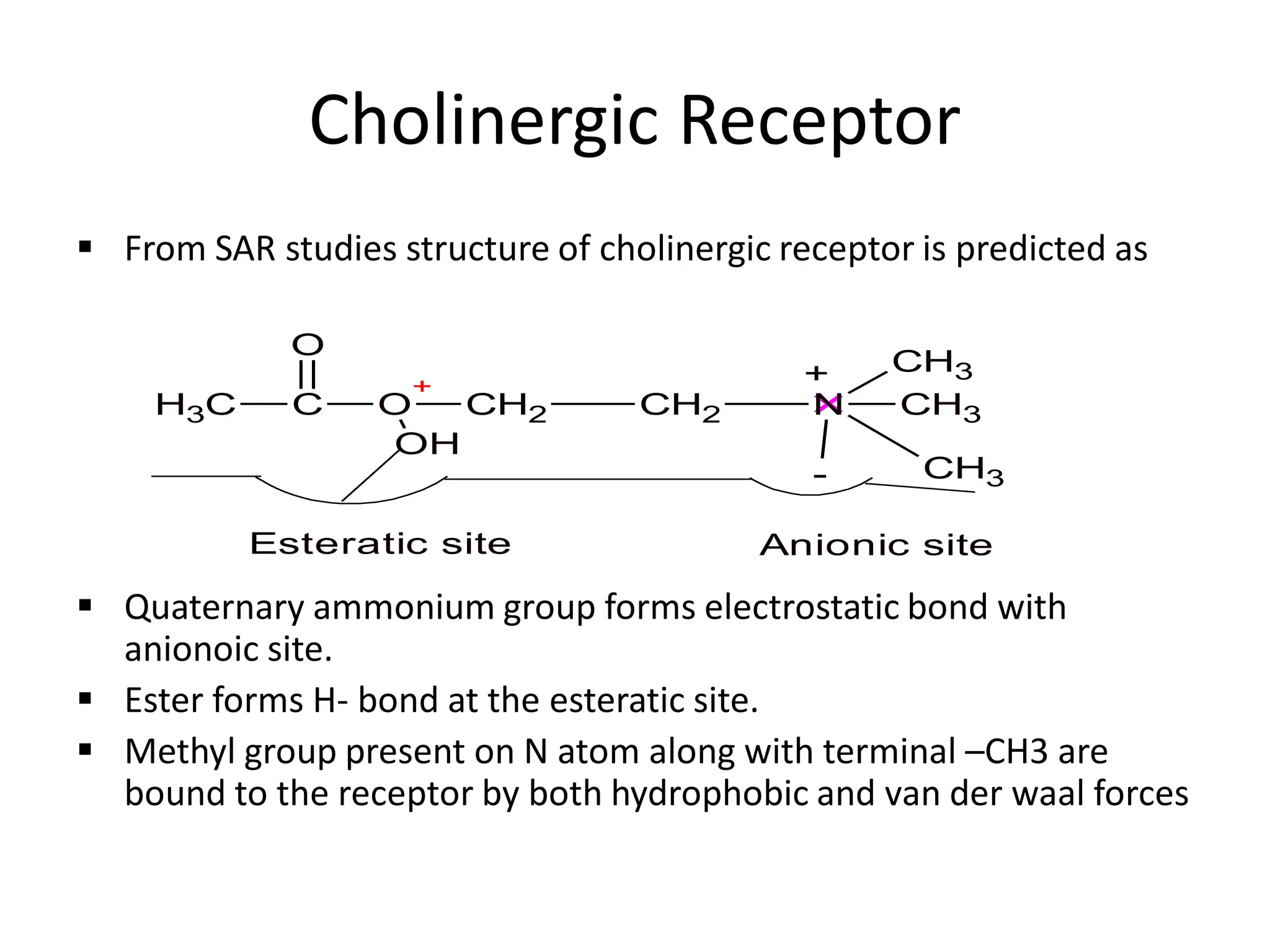
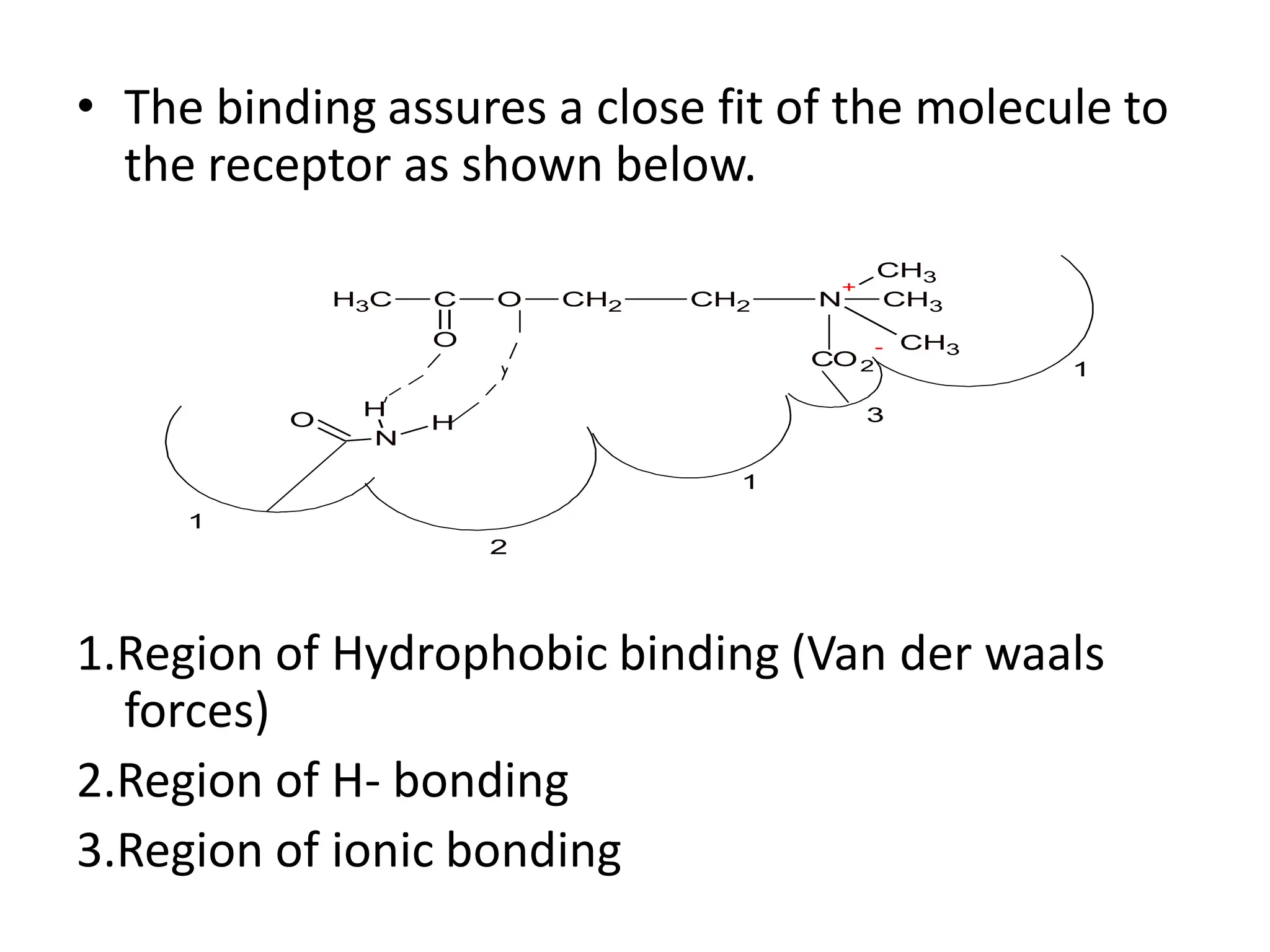
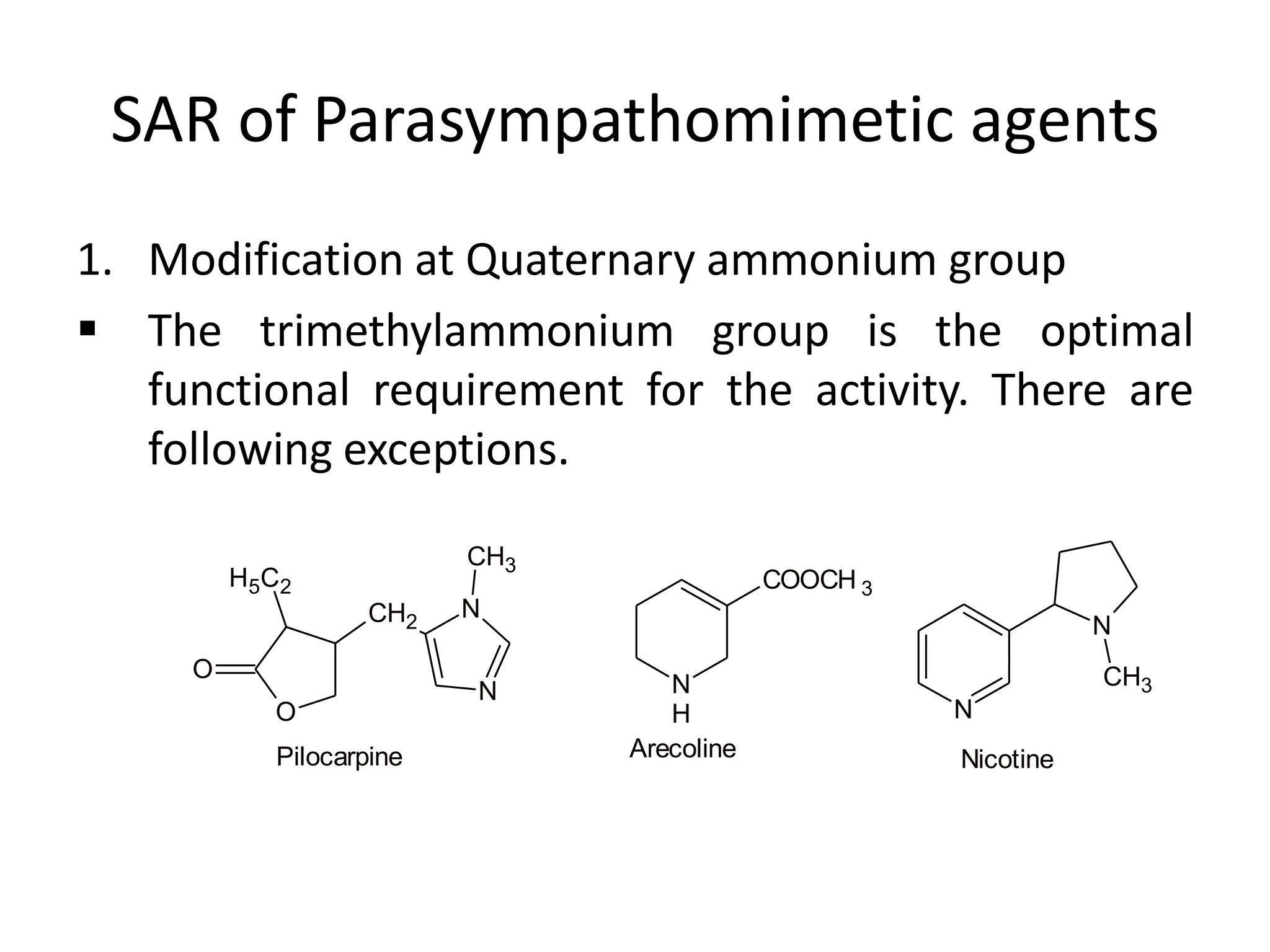
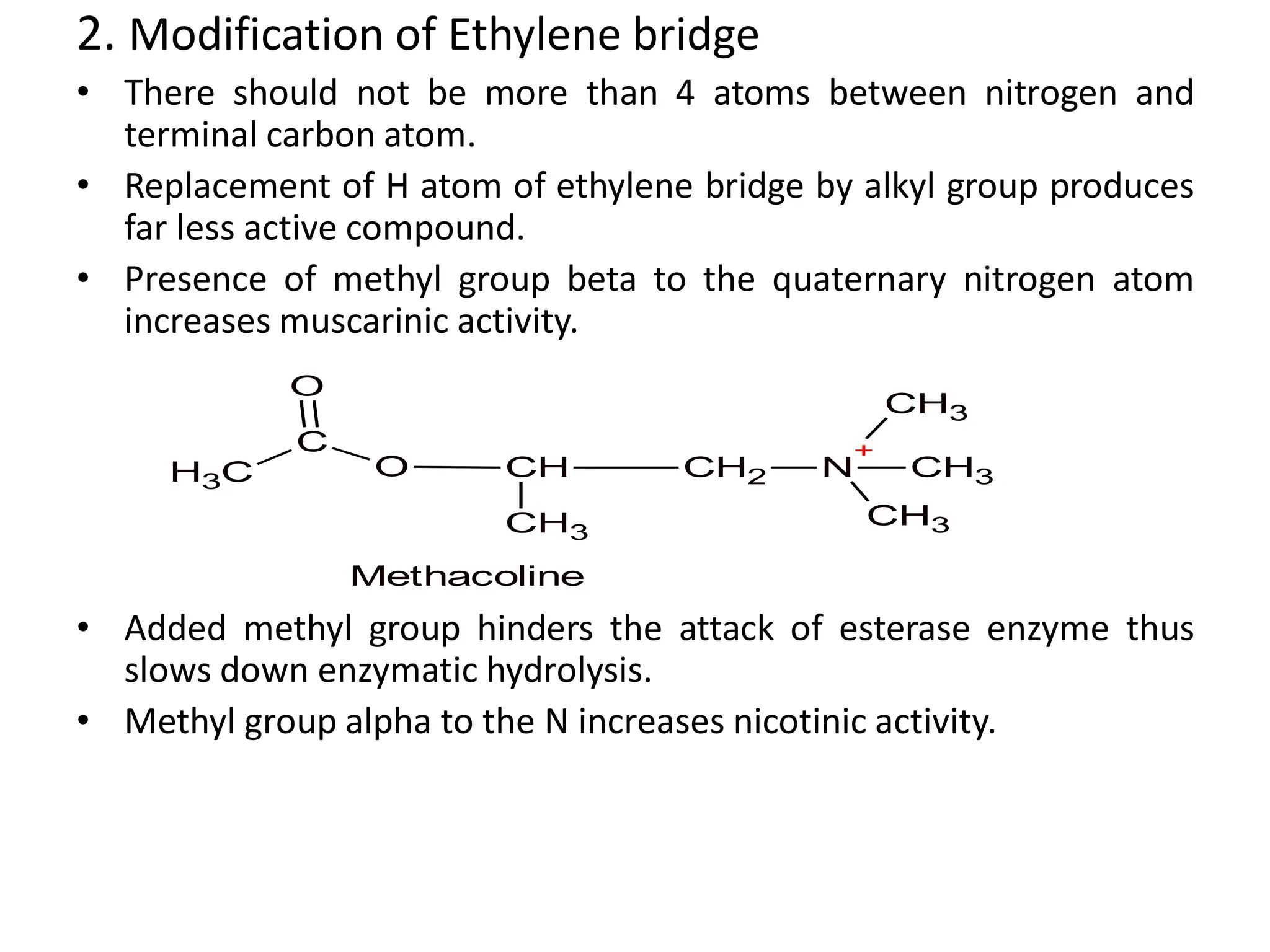
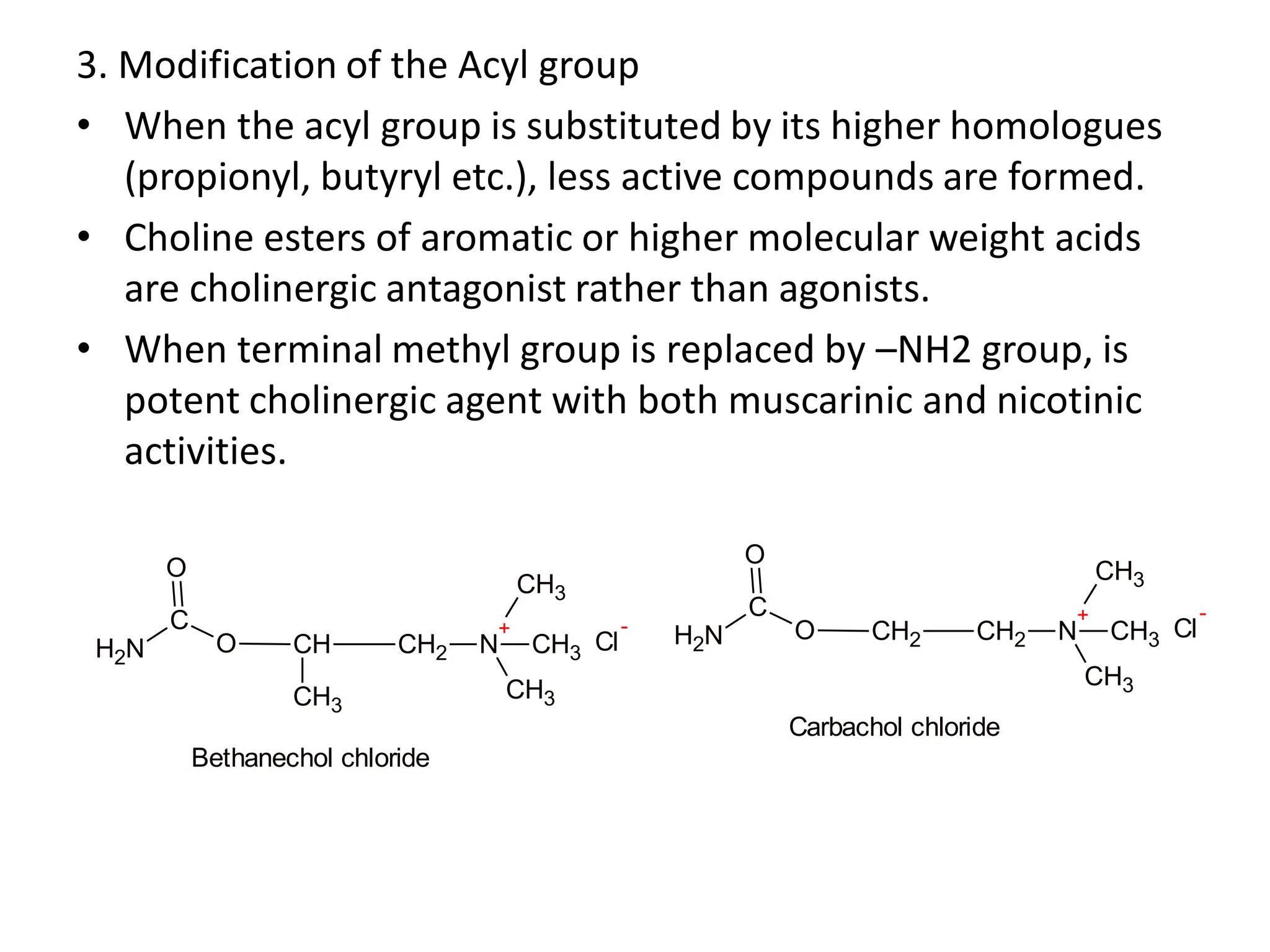
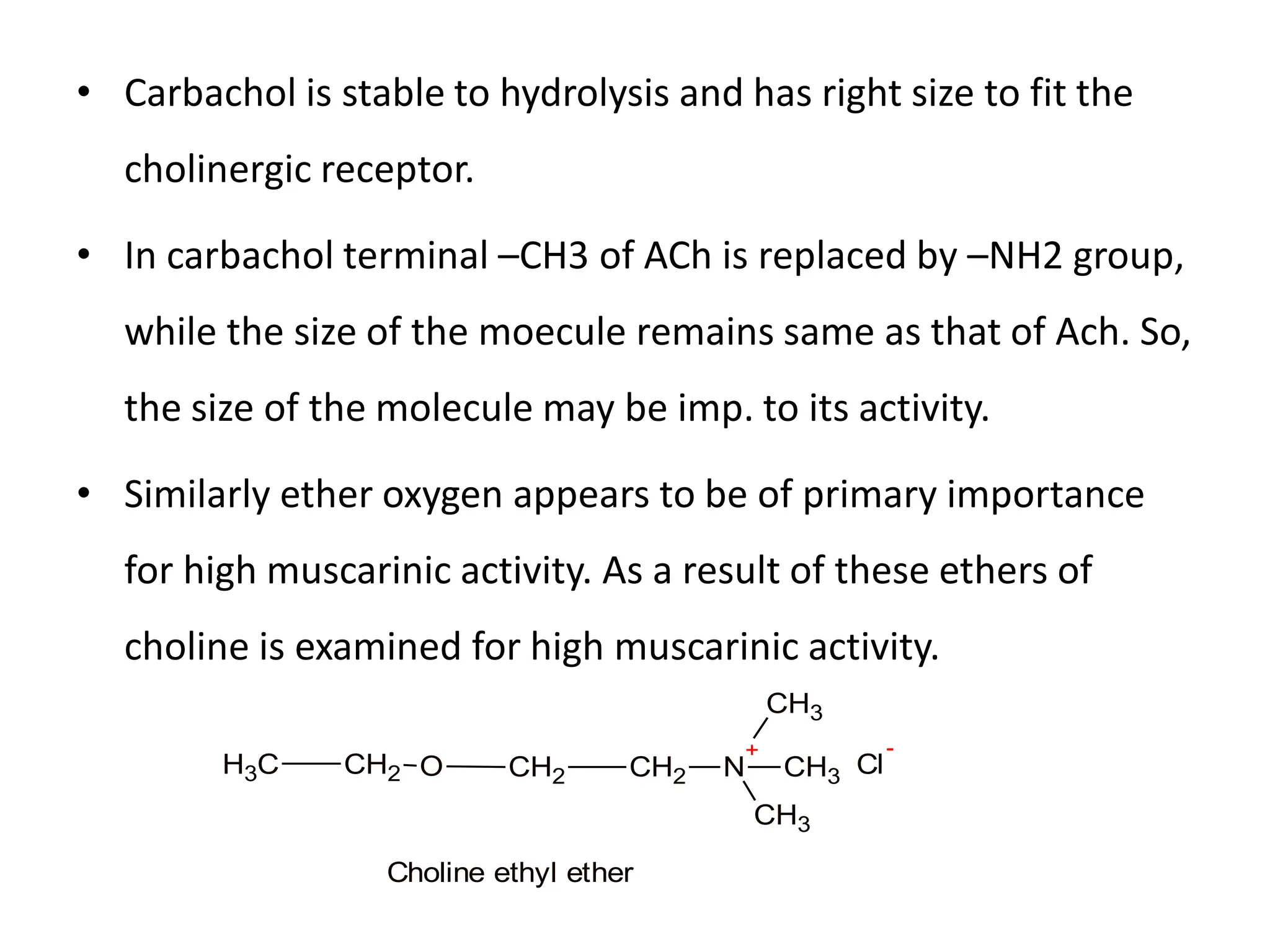
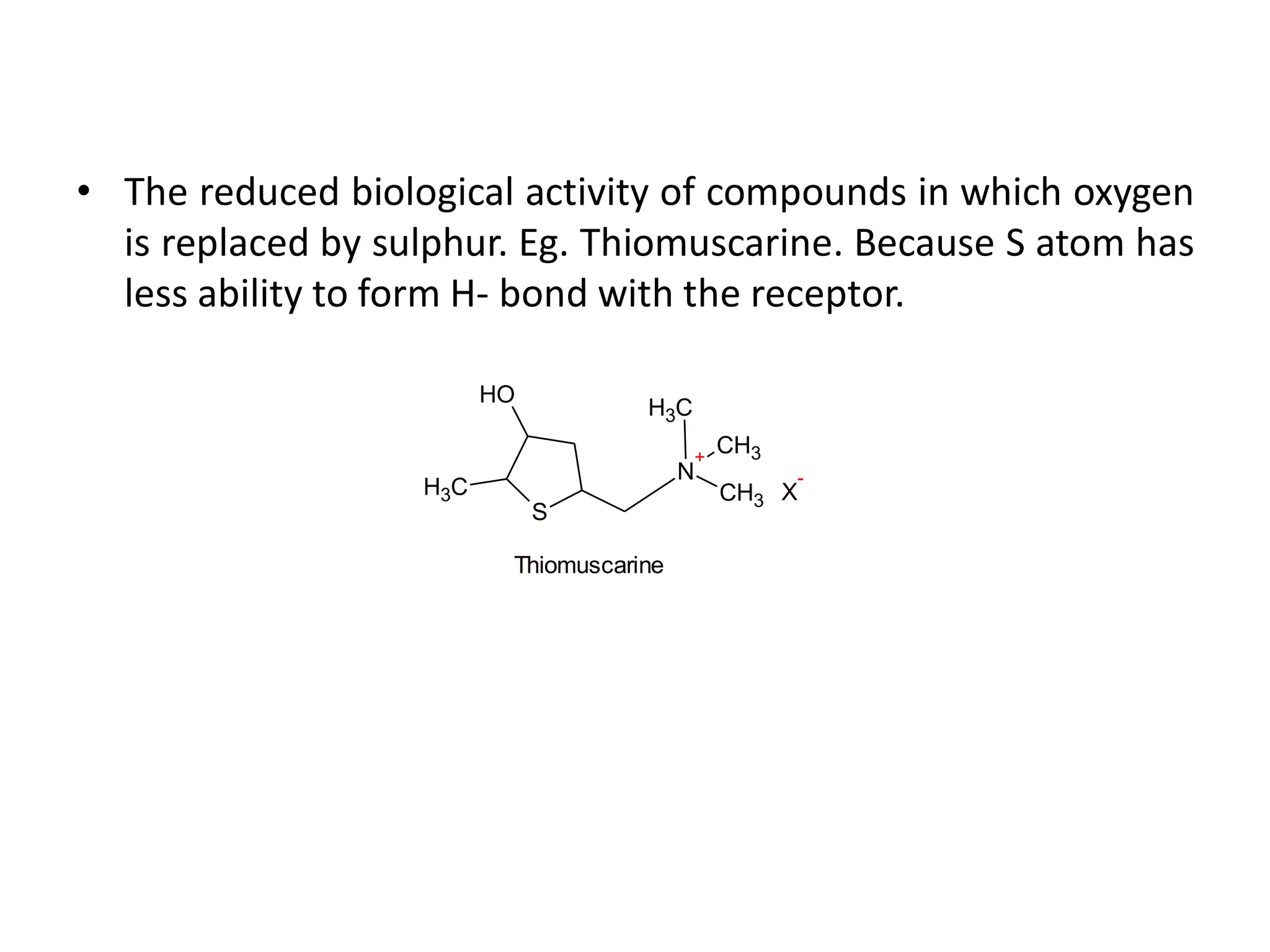
The document provides an overview of the cholinergic nervous system, focusing on the neurotransmitter acetylcholine (ACh) and its effects on the parasympathetic nervous system. It details the biosynthesis and release of ACh, its interactions with various types of receptors (nicotinic and muscarinic), and chemical characteristics essential for receptor activity. Additionally, it discusses modifications to ACh that can alter its pharmacological effects and the stability of cholinergic agents.