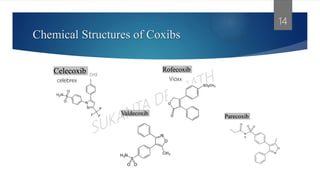
The presentation discusses cyclooxygenase (Cox) enzymes, specifically Cox-2 inhibitors, which are nonsteroidal anti-inflammatory drugs that target Cox-2 to alleviate pain and inflammation. It explores the mechanisms of action, types of Cox enzymes, categories of NSAIDs, as well as the side effects and selective targeting of Cox-2 inhibitors. Additionally, it highlights the structure and classification of non-selective Cox-2 inhibitors and provides examples and references for further study.