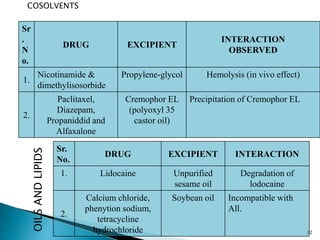
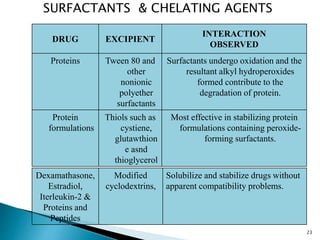
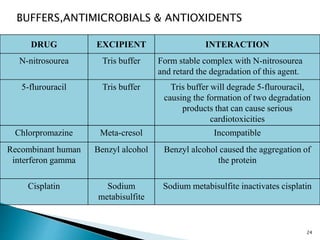
The document discusses the critical importance of drug excipient compatibility in preformulation stages of dosage development, emphasizing various types of interactions and their effects on drug stability. It outlines the objectives of compatibility studies, methodologies for testing, and the impact of excipient properties on drug degradation mechanisms, including pH sensitivity and moisture absorption. Additionally, it provides examples of how specific excipients can destabilize certain drugs and highlights the role of statistical designs in evaluating compatibility in complex formulations.