The document provides a detailed manual for performing a Complete Blood Count (CBC) using various diluents and methods to count white blood cells (WBCs), red blood cells (RBCs), and platelets. It outlines procedures for sample collection, reagent preparation, and the use of a hemocytometer for accurate cell counting, as well as calculations for determining cell concentrations. Specific notes on special cases and conditions affecting cell counts are also included.

![REAGENTS & EQUIPMENTS
1. Sample:
- EDTA whole blood sample.
- Fresh blood direct from patient.
2. Reagents:
- Platelets: Ammonium oxalate (1%).
- WBCs: Glacial acetic acid (2%).
- RBCs: Saline (0.9%).
3. Equipment's:
- Microscope.
- Hemocytometer (improved Neubauer )+ its cover slide.
- Pipette [10ul, 20ul, 100-1000 variable].
- Petri dish lined with filter paper that has been moistened.
- Hand counter.
- Rack and tubes.](https://image.slidesharecdn.com/practicalhematologyl01manualcbc-230707133936-6fe5f721/85/Practical-Hematology-l-01-Manual-CBC-pdf-2-320.jpg)



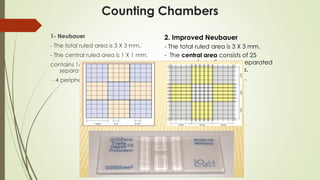









![Red Blood Cells (RBCs) count
Time-consuming & so imprecise [Obsolete]
Sample:
- EDTA blood or
- Directly from the patient finger.
Diluent:
- Saline (0.9%).
- Formal citrate (10 ml formalin +1 L of trisodium citrate).
Which is better formal citrate or saline ?
- Formal citrate is better than saline as you can prepare the dilution and leave
The sample without change in the shape of RBCs but if you use saline, you must examine the sample as soon as possible.
Dilution: 1:200
- 20 µl blood + 4 ml (= 4000ul) saline or formal citrate and mix well.
Steps:
1. Adjust the hemocytometer on the microscope as before.
2. Fill the hemocytometer with diluted blood.
3. Count in the same squares as platelets](https://image.slidesharecdn.com/practicalhematologyl01manualcbc-230707133936-6fe5f721/85/Practical-Hematology-l-01-Manual-CBC-pdf-16-320.jpg)

![Calculation:
كله االوسط المربع حجم
=
الطول X العرض X االرتفاع
= 0.1
في العد ان بما
5
من مربعات
25
مربع
= 1/5
= 0.02 = [1/10 ml X 1/5]
RBCs count = No. of cells counted in 5 squares X Dilution.
Volume of 5 squares
RBCs count = (No. of cells counted ⁄ 0.02 ) X 200.
= Count X 10,000.](https://image.slidesharecdn.com/practicalhematologyl01manualcbc-230707133936-6fe5f721/85/Practical-Hematology-l-01-Manual-CBC-pdf-18-320.jpg)




![Calculation:
كله األوسط المربع حجم
=
الطول
X
العرض
X
االرتفاع
=
0.1
في العد ان بما
5
من مربعات
25
مربع
=
1/5
=
0.02 = [0.1ml X (1/5)]
PLTs count =No.of cells counted in 5 squares X Dilution.
Volume of 5 squares
PLTs count = (No. of cells counted ⁄ 0.02 ) X 20.
= [40 + 42 + 40 + 38 + 36]
= Count X 1000 = 200 X 1000 =
= 200,000](https://image.slidesharecdn.com/practicalhematologyl01manualcbc-230707133936-6fe5f721/85/Practical-Hematology-l-01-Manual-CBC-pdf-23-320.jpg)








