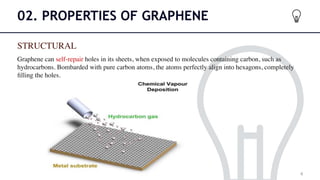
This document provides an introduction to graphene through a seminar presentation. It defines graphene as a pure carbon material made of a single layer of carbon atoms arranged in a hexagonal lattice. The presentation summarizes some of graphene's key properties including its strength, flexibility, electrical and thermal conductivity. It also outlines several methods for producing graphene, such as mechanical exfoliation and reduction of graphite oxide. Finally, the document discusses potential applications of graphene in areas like solar cells, batteries, electronics and aerospace.