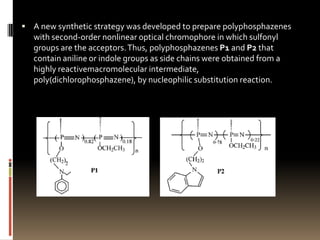
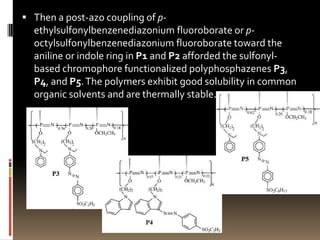
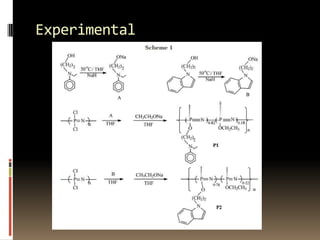
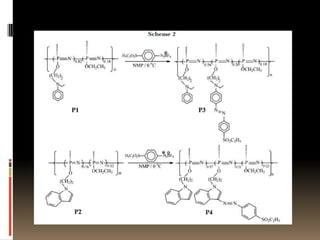
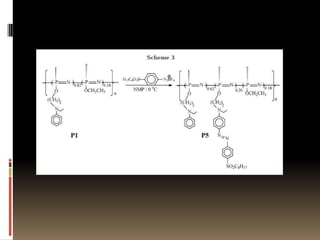
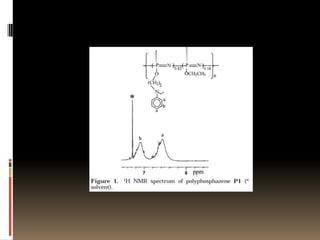
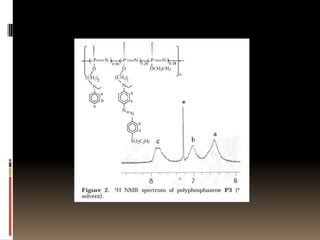
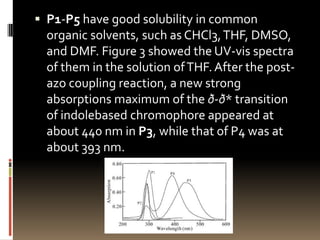
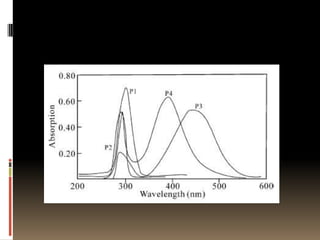
This document describes a new synthetic strategy for preparing polyphosphazene polymers with second-order nonlinear optical properties. The strategy involves first producing polyphosphazenes containing aniline or indole side chains, then performing a post-azo coupling reaction to attach sulfonyl-based chromophores to the side chains, yielding polymers P3, P4, and P5. These polymers exhibit good solubility and thermal stability, with absorption maxima blue-shifted compared to similar chromophores containing nitro groups. Poled films of P3 and P4 showed second-order nonlinear optical coefficients of 27 and 18 pm/V, respectively.