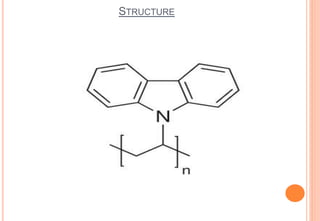
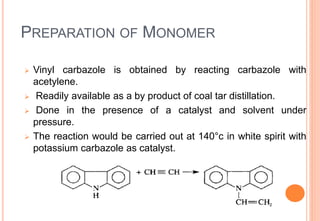
Poly(vinyl carbazole) is known as a photoconductive material whose electrical conductivity increases with light exposure. It has good heat and chemical resistance and softens at 150°C. The monomer vinyl carbazole is prepared through a reaction of carbazole with acetylene and polymerized using initiators like di-tert-butyl peroxide. Poly(vinyl carbazole) is insoluble in many solvents and difficult to process, requiring high temperatures of 300°C for injection molding. Its main application is in electrostatic dry copying machines where it is used as the photoconductive layer in xerography.