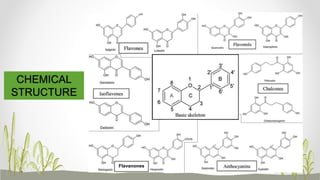
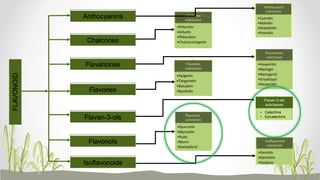
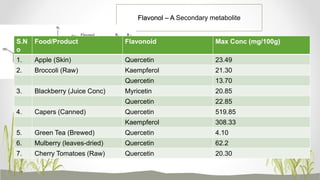
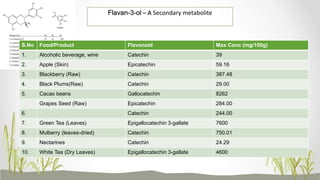
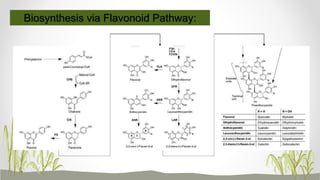
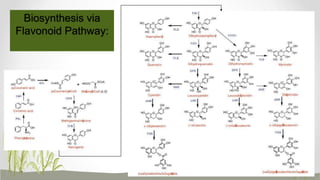
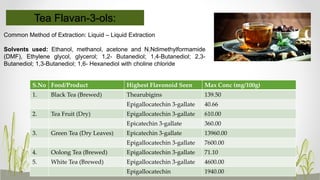
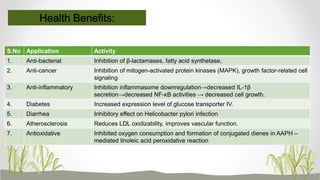
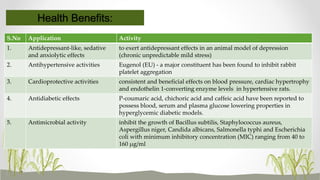
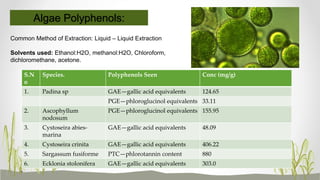
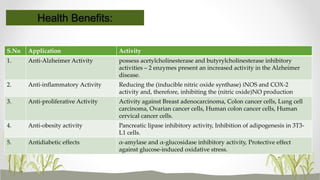
This document discusses plant polyphenols, which are secondary metabolites found in plants. It focuses on polyphenols found in tea, mulberry, tulsi, algae, and their extraction methods, highest concentrations in foods, and health benefits. Some key applications of polyphenols discussed are their anti-bacterial, anti-cancer, anti-inflammatory, and antioxidant properties. Analytical instrumentation used to study polyphenols includes HPLC/MS, GC/MS, and NMR spectroscopy. The conclusion emphasizes the importance and therapeutic potential of plant polyphenols.