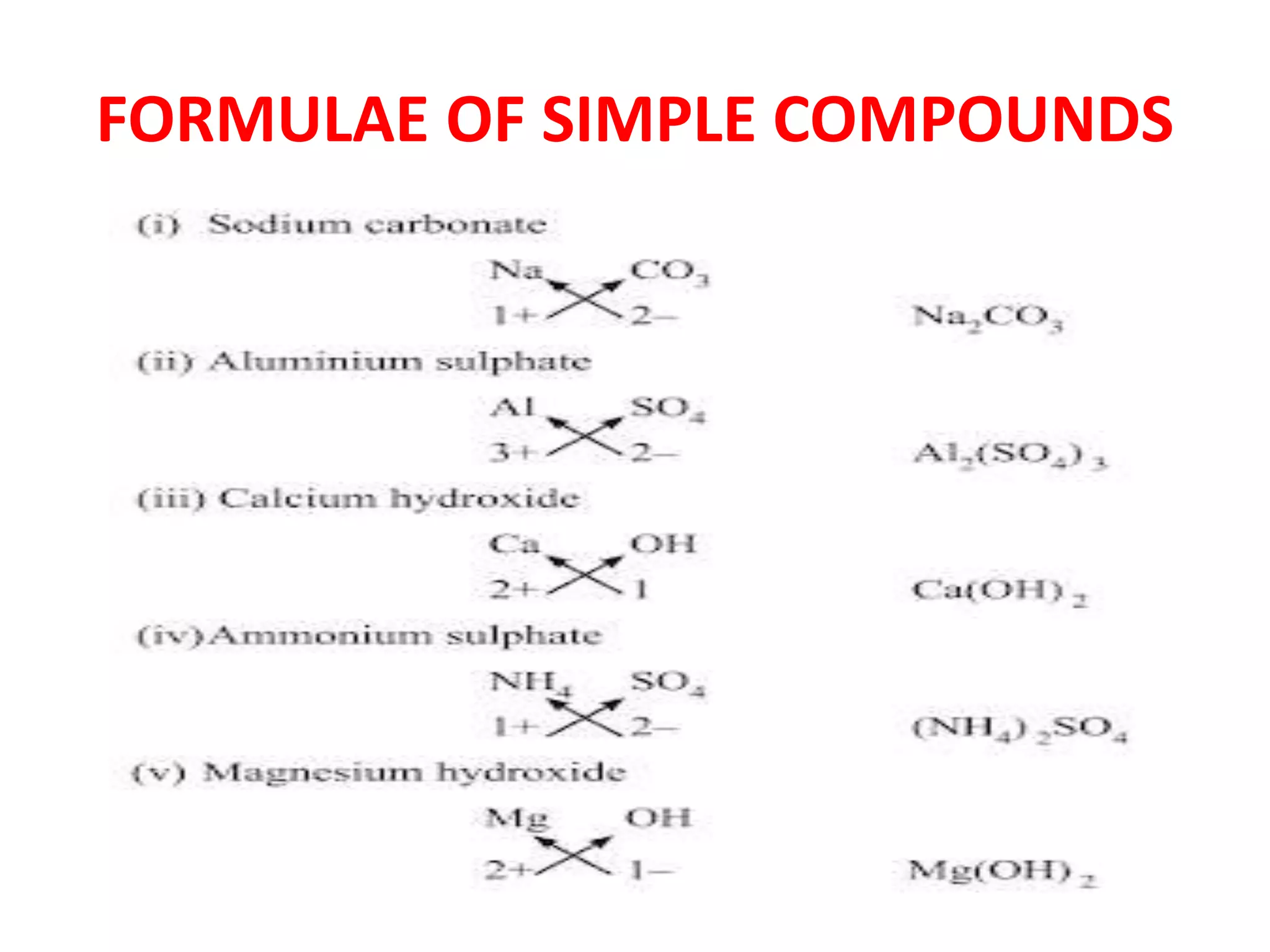
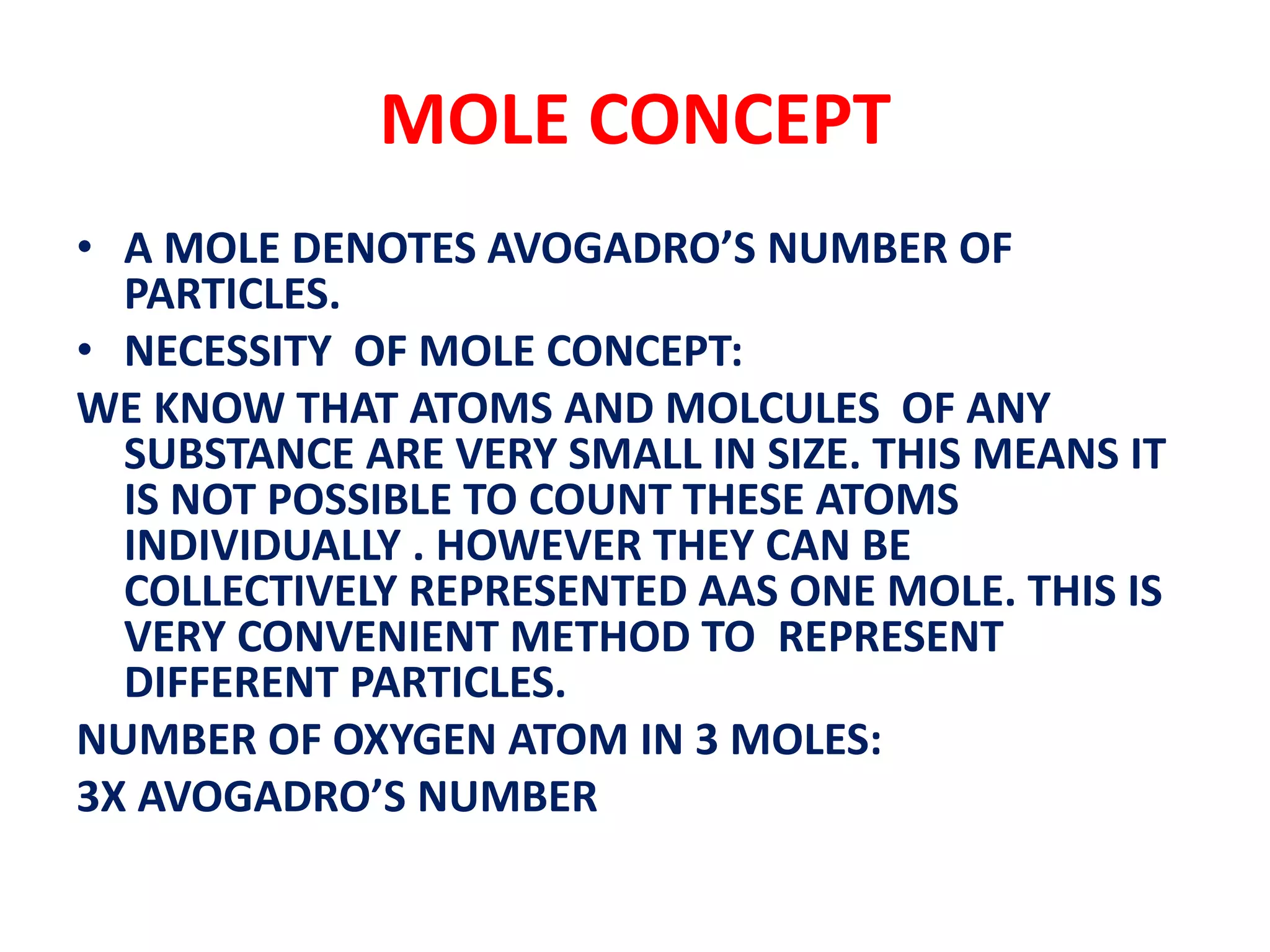
This document discusses molecules, atoms, and the mole concept in chemistry. It defines molecules as combinations of two or more atoms, and describes molecules of elements and compounds. Molecules can be diatomic, triatomic, tetra-atomic, or poly-atomic depending on the number and type of atoms combined. The mole concept is introduced as a convenient way to represent large numbers of particles collectively. One mole equals Avogadro's number of particles, and conveys information about number, mass, and volume.