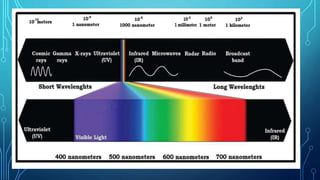
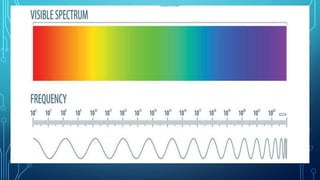
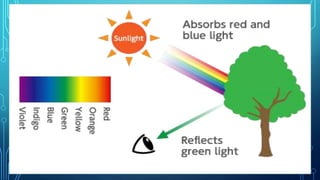
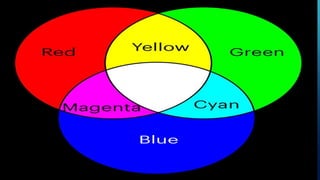
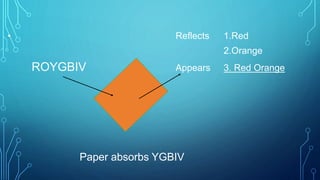
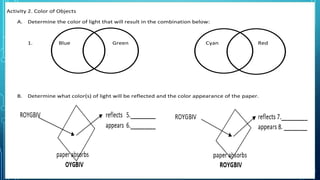
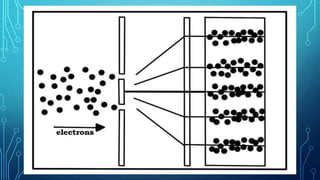
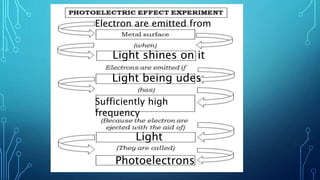
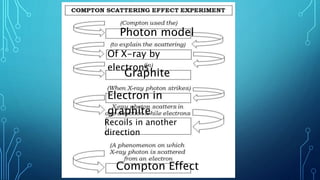
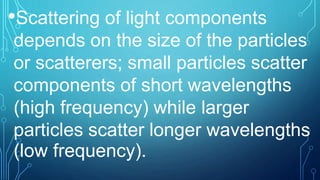
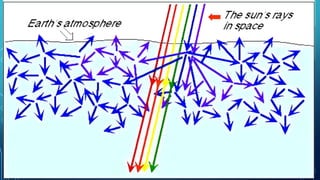
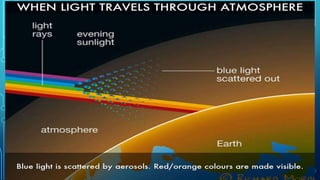
Here are the answers to the scatter-hunting activity:
1. Rayleigh scattering
2. Mie scattering
3. Mie scattering
4. Rayleigh scattering
5. Rayleigh scattering
6. Rayleigh scattering
7. Rayleigh scattering
8. Mie scattering
9. Mie scattering
10. Rayleigh scattering