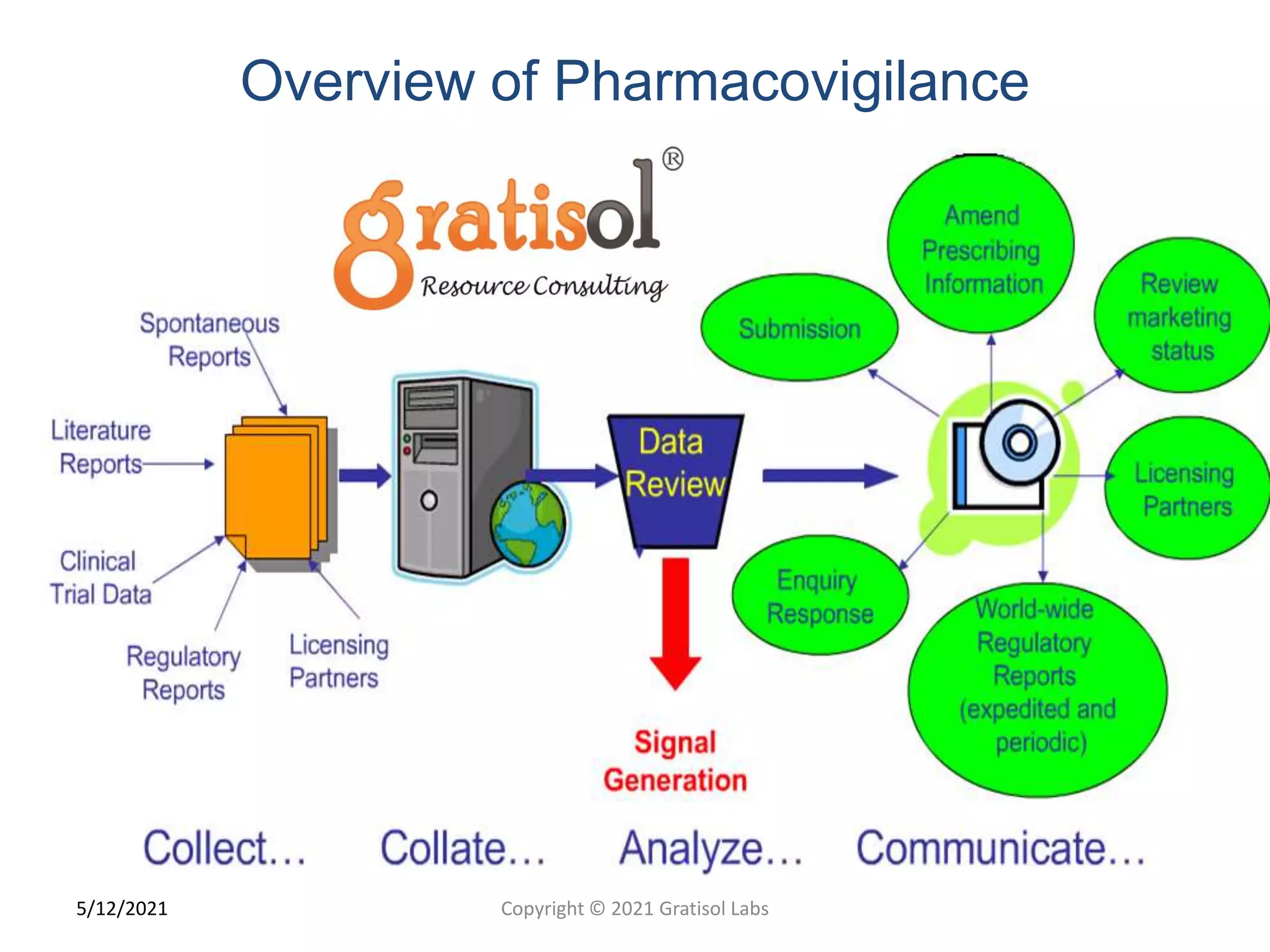
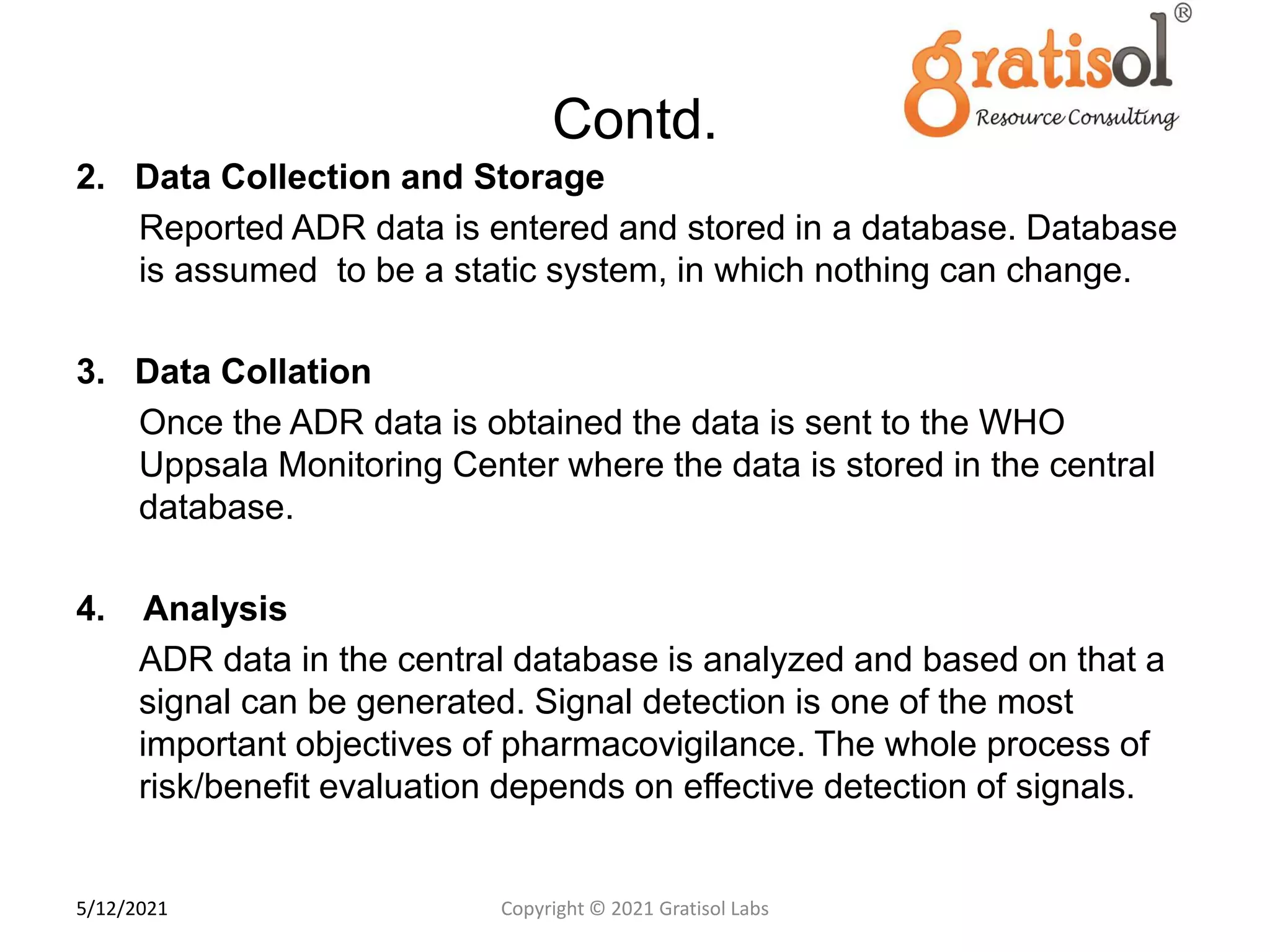
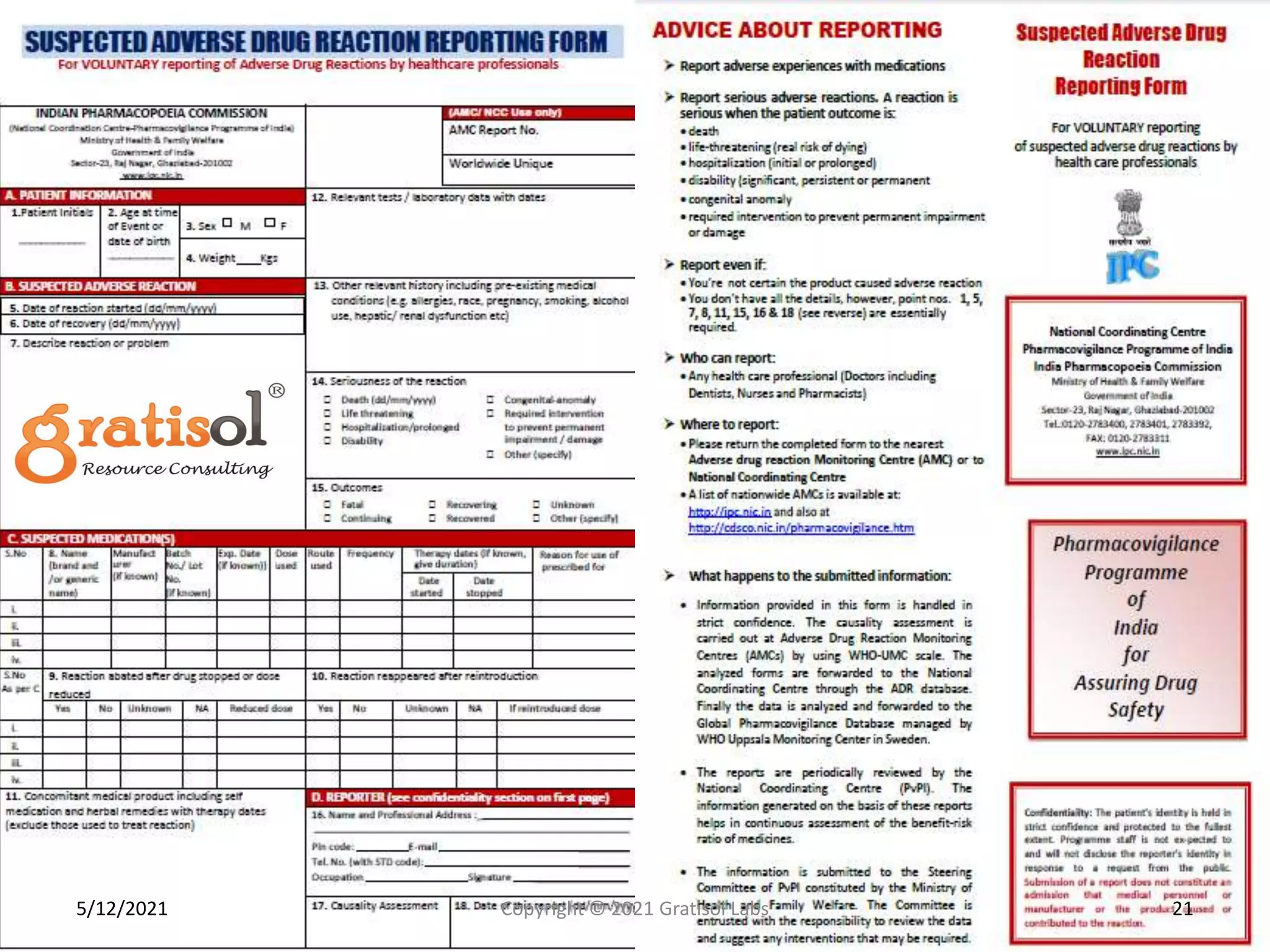
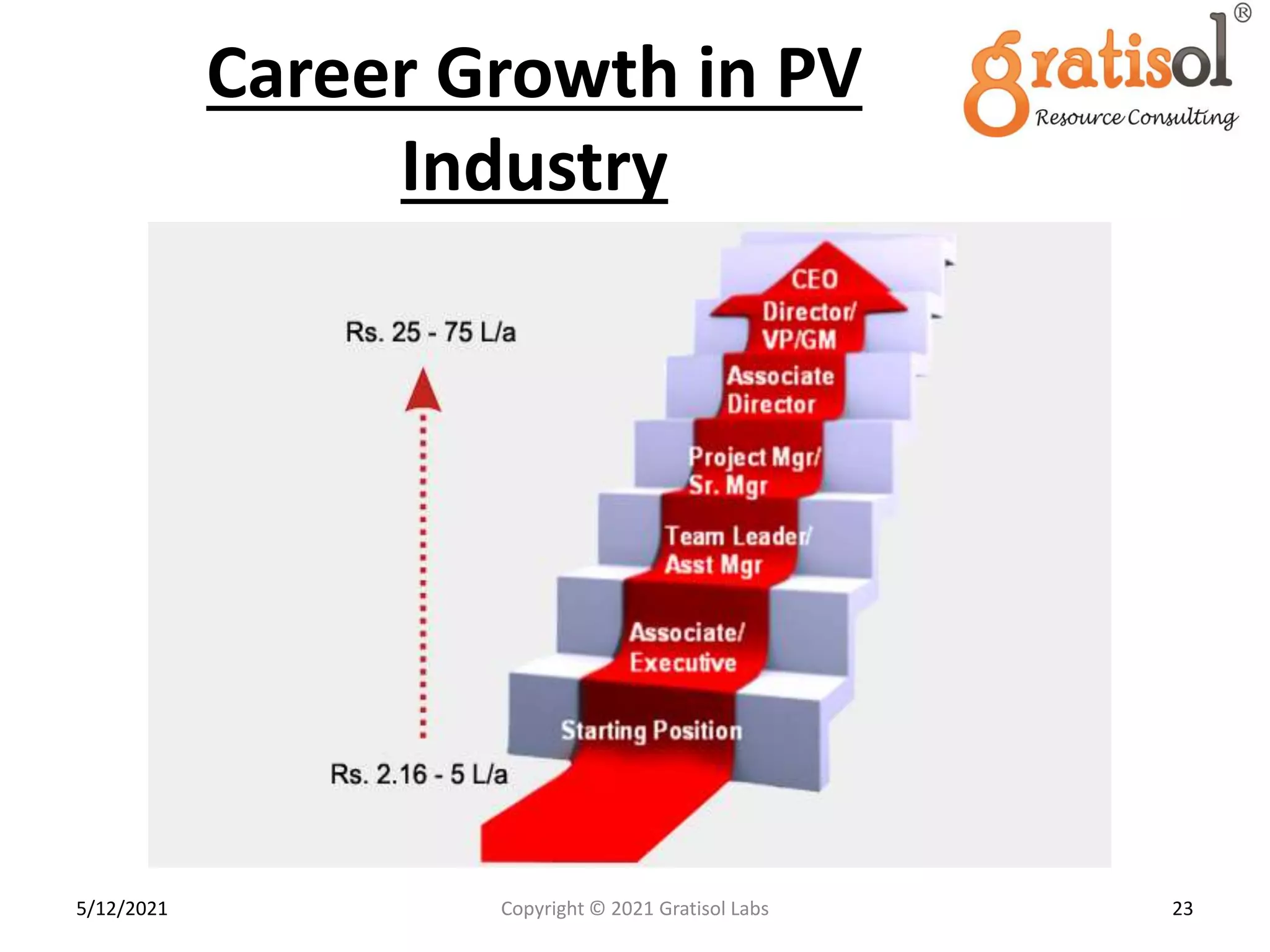
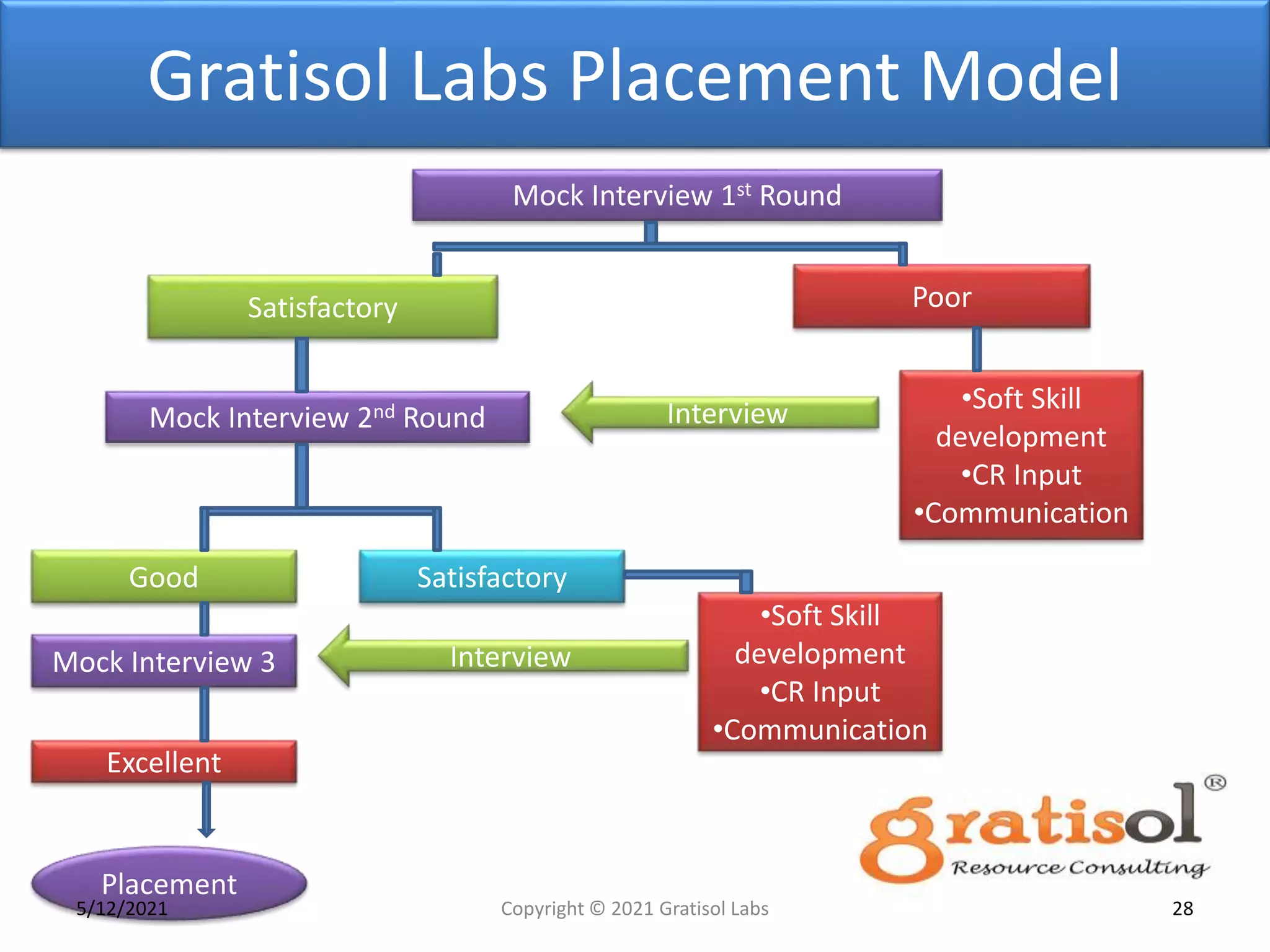
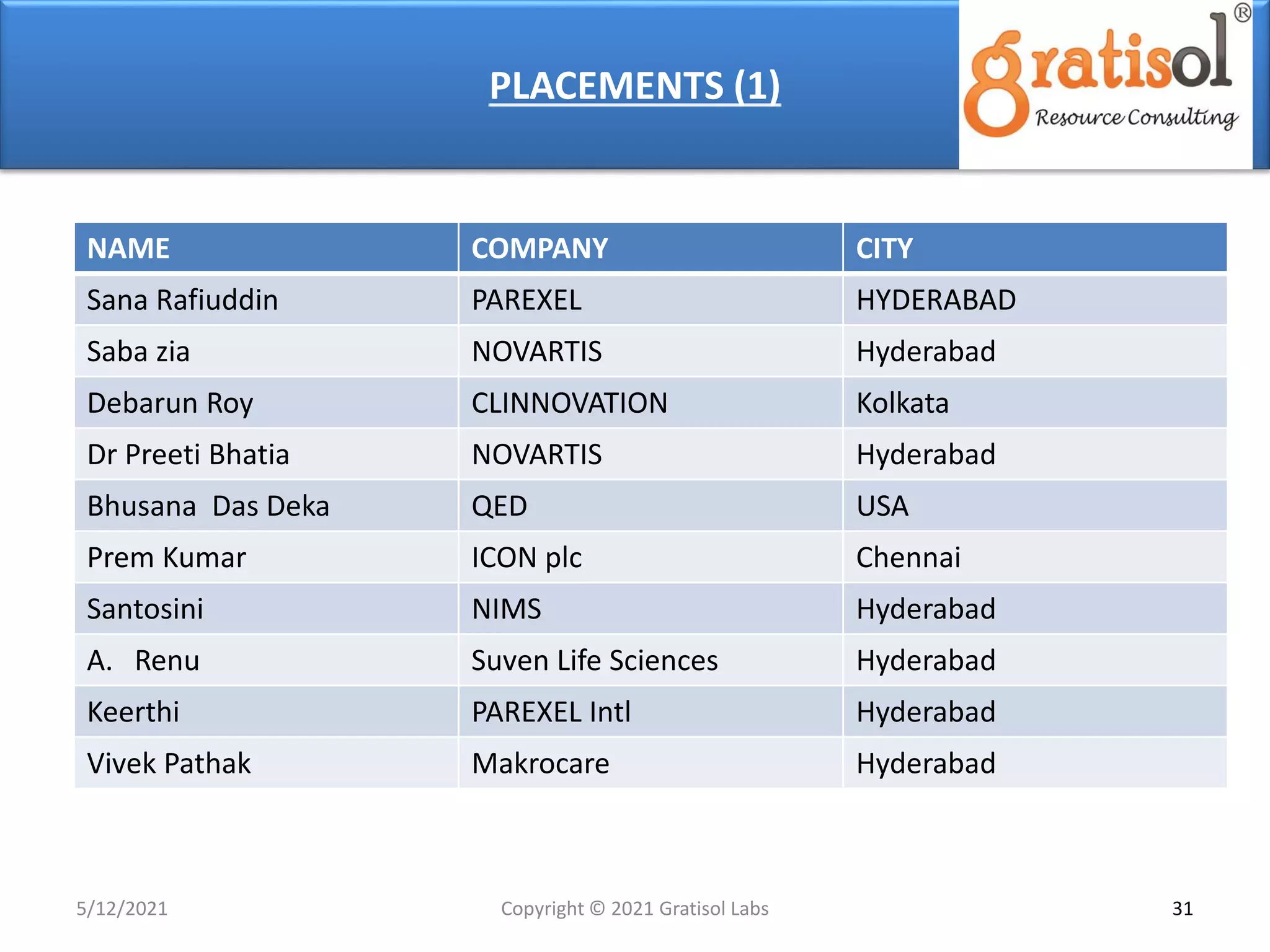
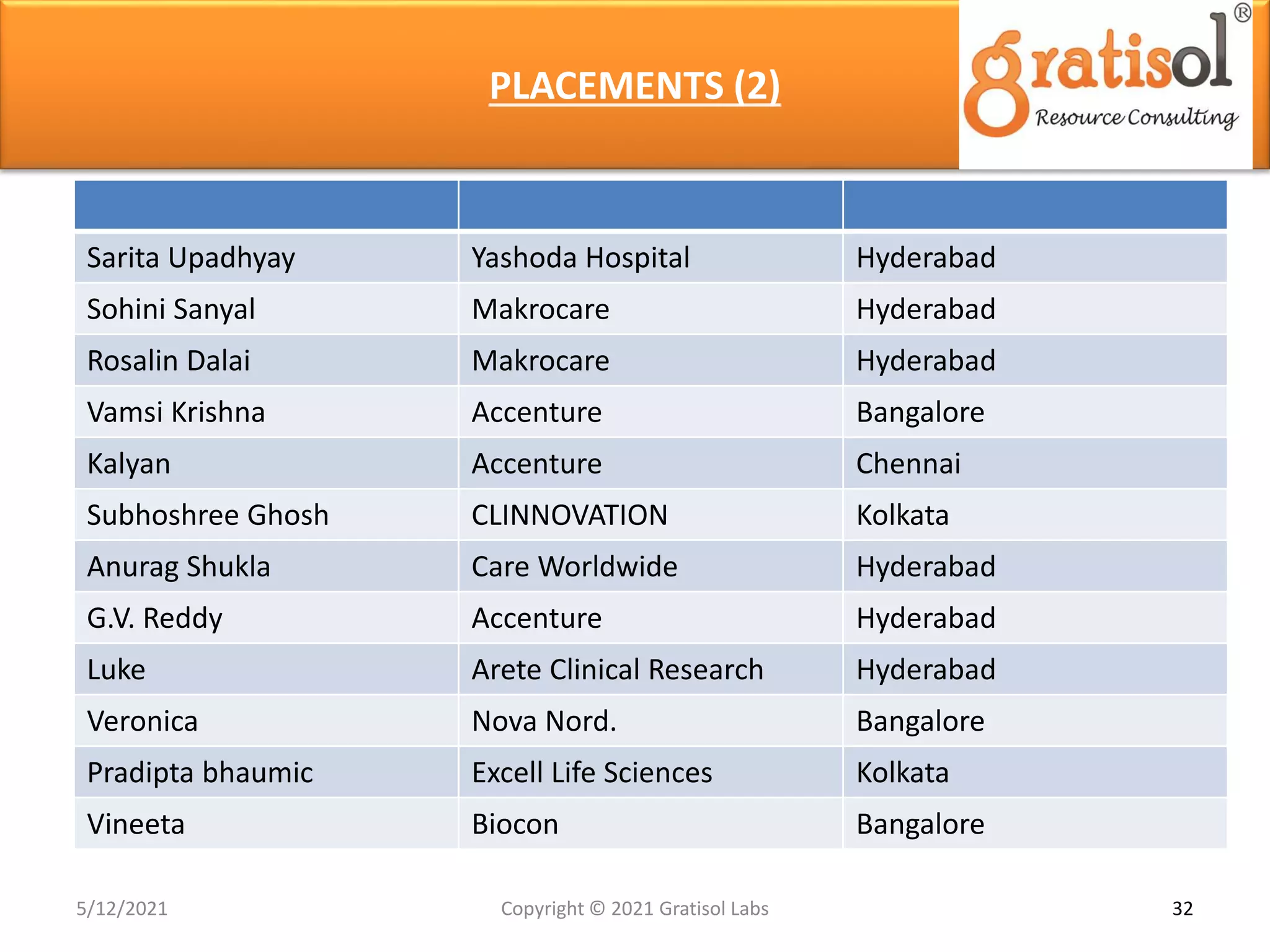
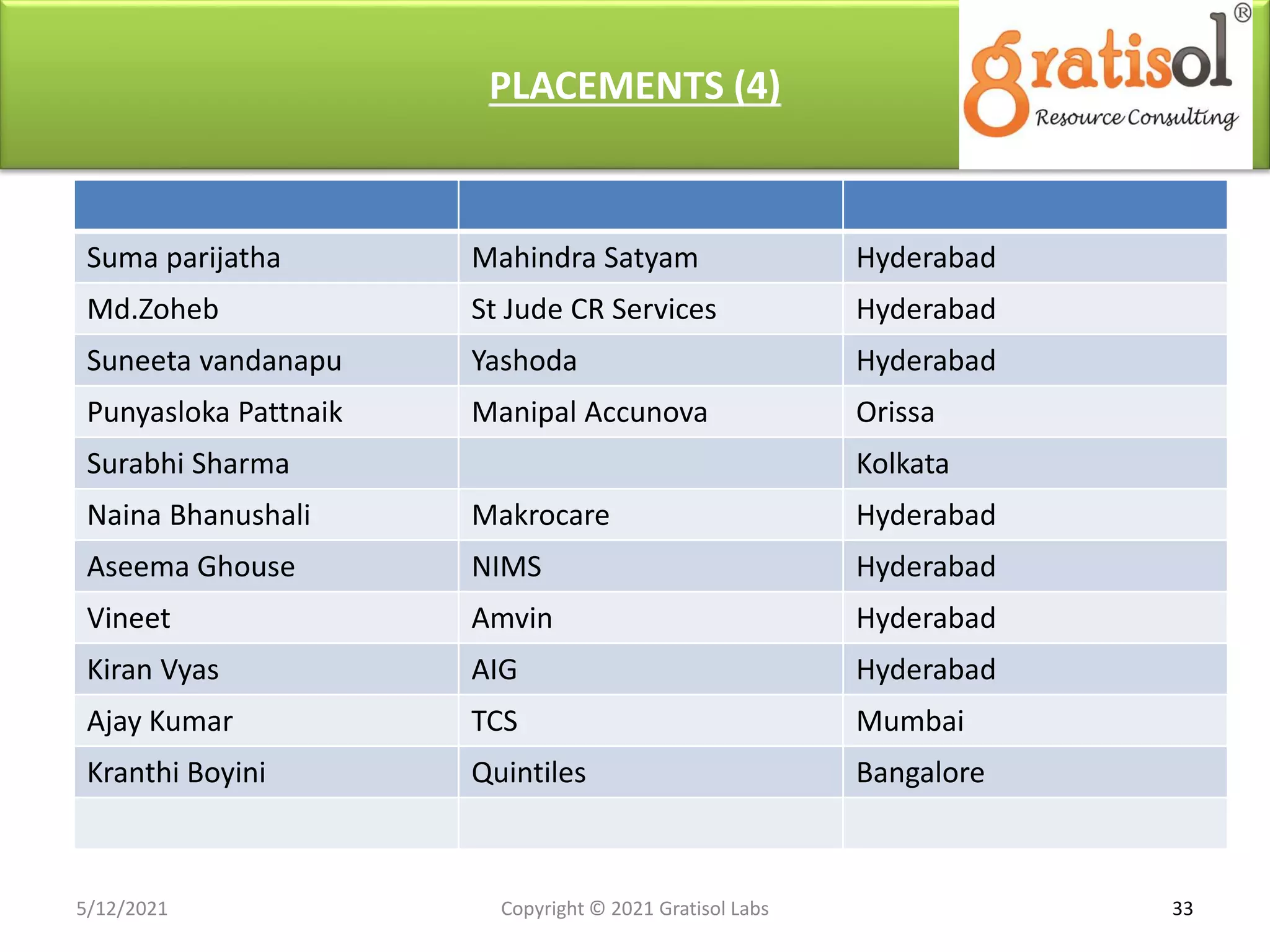
Gratisol Labs is a prominent training institute specializing in pharmaceutical sciences, offering various programs including pharmacovigilance training essential for monitoring drug safety and compliance with regulatory guidelines. The document outlines the importance of pharmacovigilance, key definitions, and processes involved in reporting and analyzing adverse drug reactions. It also discusses career prospects in the field, emphasizing the growing demand for trained professionals in pharmacovigilance.