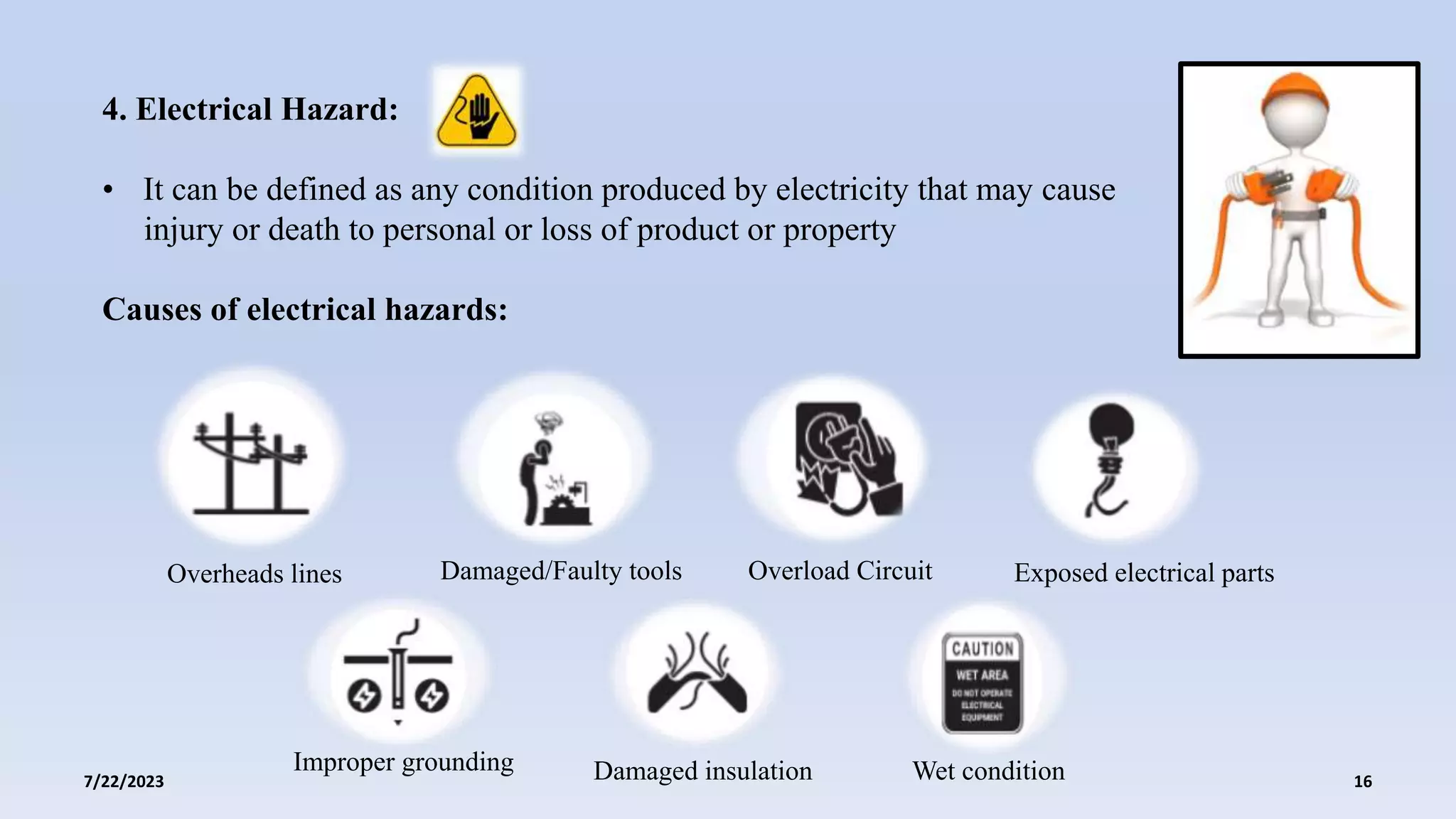
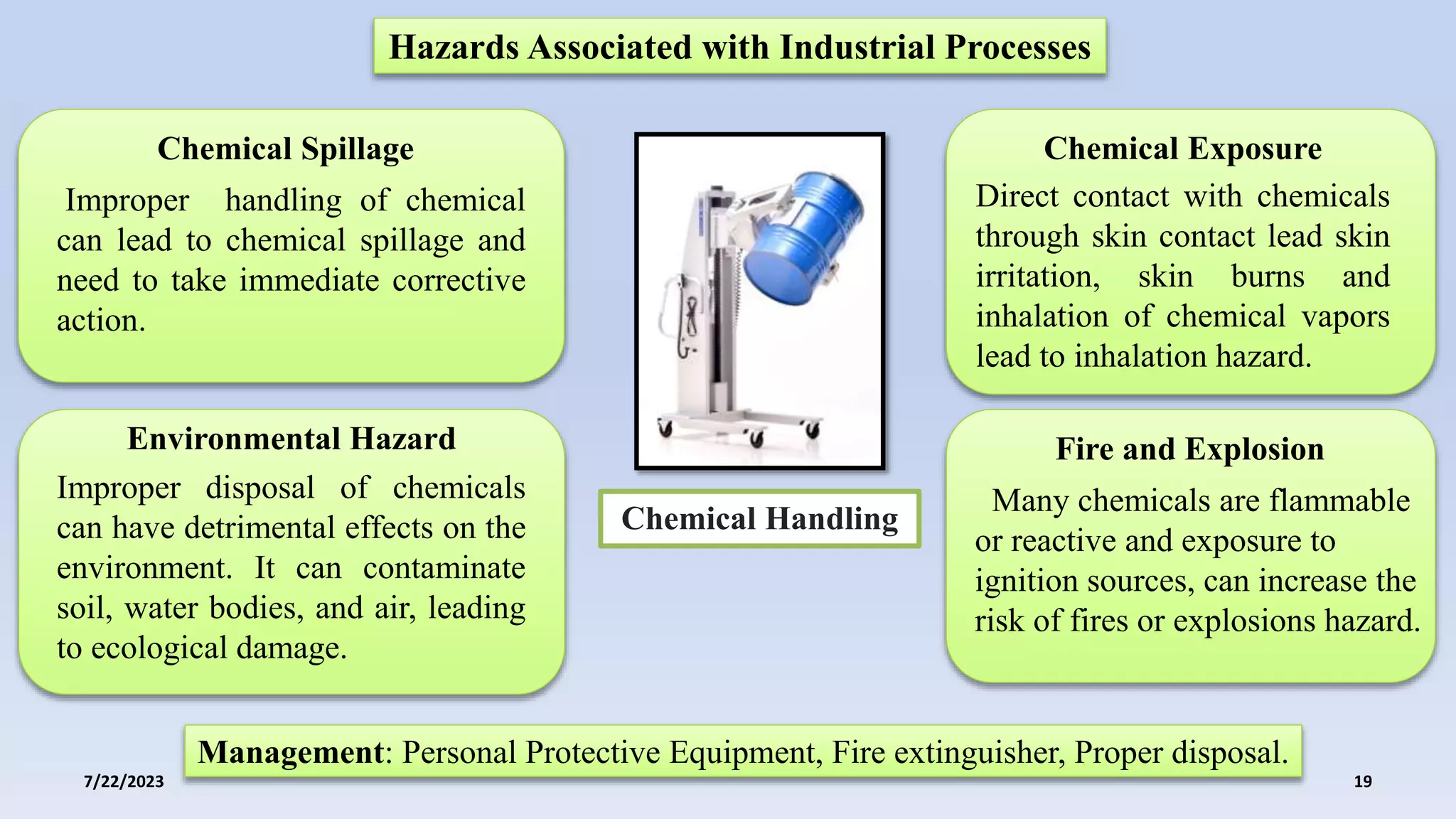
The document discusses the potential hazards associated with industrial processes in the pharmaceutical sector, including physical, chemical, biological, electrical, ergonomic, and thermal hazards. It emphasizes the importance of risk assessment, safety protocols, and compliance with regulations to mitigate risks and ensure the safety and efficacy of pharmaceutical products. Case studies illustrate the consequences of improper hazard management and highlight the need for continual improvement in safety practices.