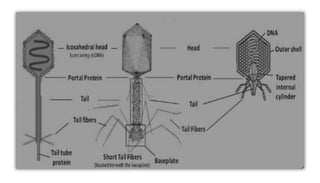
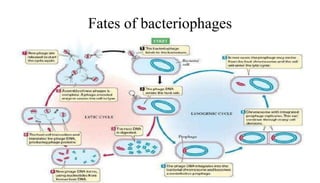
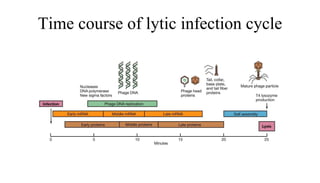
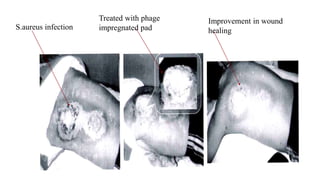
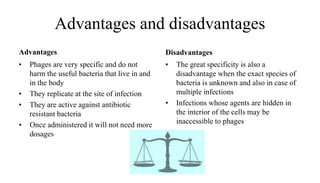
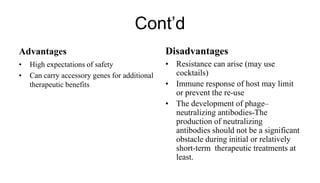
This document provides an overview of phage therapy. It discusses bacteriophages and their structure. It covers the growing problem of antibiotic resistance and how phage therapy could provide an alternative treatment. The history of phage therapy is reviewed, along with initial problems, solutions, administration examples, and future implications. Both advantages and disadvantages of phage therapy are presented. The document concludes that modern innovations in phage therapy combined with careful scientific methodology may help enhance its effectiveness as an alternative to antibiotics for drug-resistant bacterial infections.