Embed presentation
Download to read offline

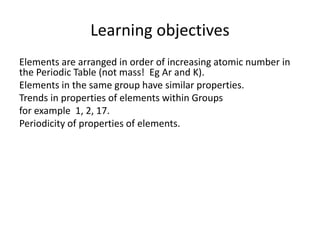
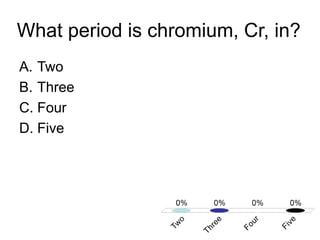
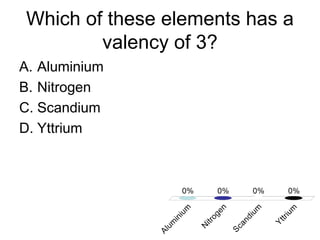
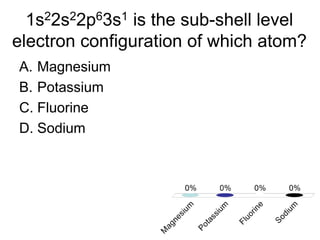
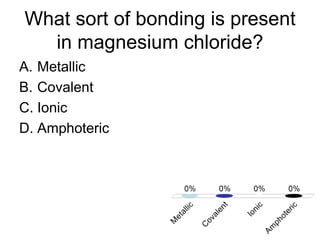
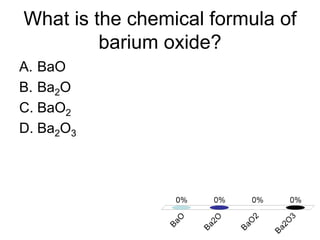

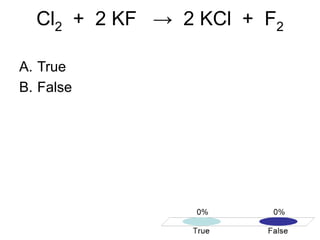


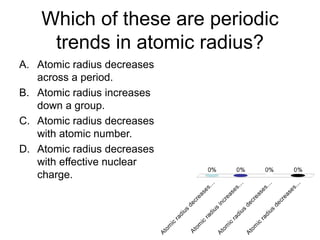

This document discusses trends in properties of elements within the periodic table. It covers which period chromium is in, which element has a valency of 3, the electron configuration of magnesium, the type of bonding in magnesium chloride, the chemical formula of barium oxide, what governs the boiling point of halogens, and periodic trends in ionization energy and atomic radius. It also mentions which atoms buck the general trend of ionization energies and asks which of the options are periodic trends in atomic radius.











