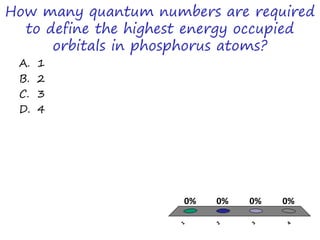
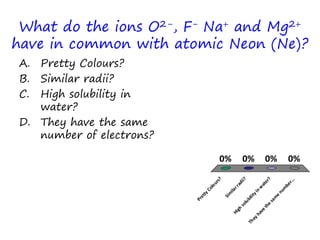
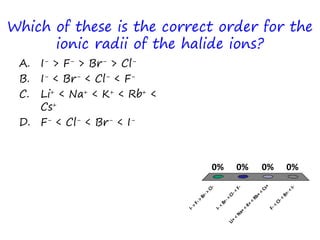
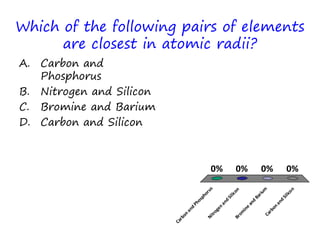
The document discusses trends in the periodic table by providing multiple choice questions about ionic radii, quantum numbers, and atomic properties. It asks about the number of quantum numbers needed to define orbitals in phosphorus atoms, how the ionic radius of O2- is known, commonalities between Neon and other ions, the order of halide ionic radii, and atomic radii of pairs of elements. The questions are intended to facilitate peer instruction on open educational resources about periodic table trends.