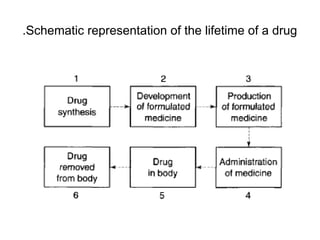
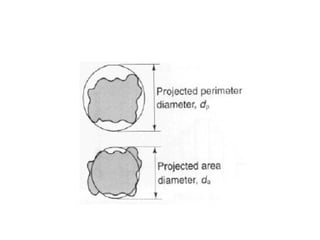
Particle-size analysis is crucial for producing effective medicines as it influences the physical and pharmacological performance of drugs. During the drug's lifetime, specifically in its synthesis, formulation, and administration, particle size affects the production process and the drug's solubility and bioavailability. While reducing particle size can enhance solubility, it may also lead to increased absorption and potential toxicity, highlighting the importance of controlled particle sizing in pharmaceuticals.