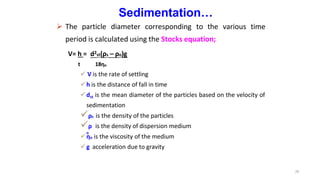
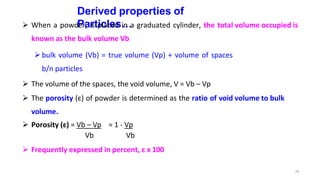
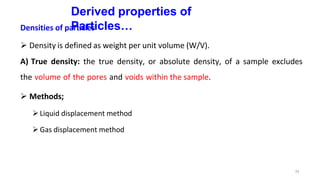
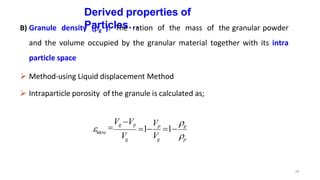
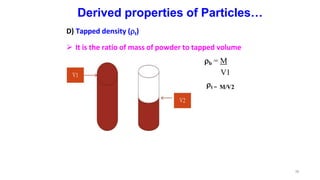
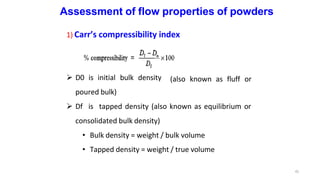
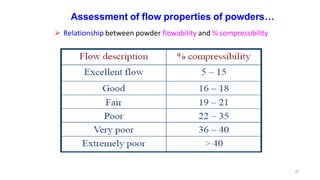
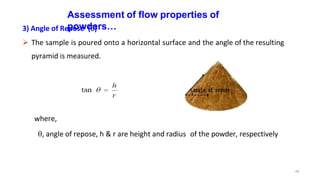
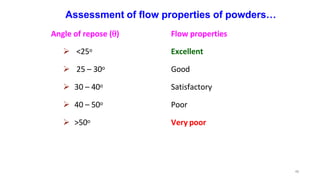
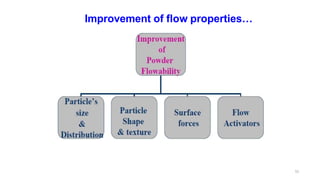
This document discusses micromeritics, focusing on the science and technology of small particles, including their size, shape, and surface area, and methods for measurement. It emphasizes the significance of these particle properties in pharmaceuticals, affecting drug formulation and performance. Additionally, the document explores derived properties such as density, porosity, and flow characteristics, along with techniques for assessing and improving powder flowability.