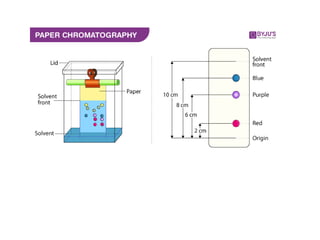
1) Paper chromatography is a technique that uses paper as the stationary phase to separate dissolved chemical substances based on their different migration rates across the paper.
2) The principle involves either partition chromatography where substances partition between the water in the paper pores and a mobile phase, or adsorption chromatography where the paper acts as a solid stationary phase and the liquid mobile phase moves through it.
3) The procedure involves selecting a paper type, preparing the sample, spotting it on the paper, developing the chromatogram by immersing the paper in a mobile phase, drying the paper, and detecting spots.