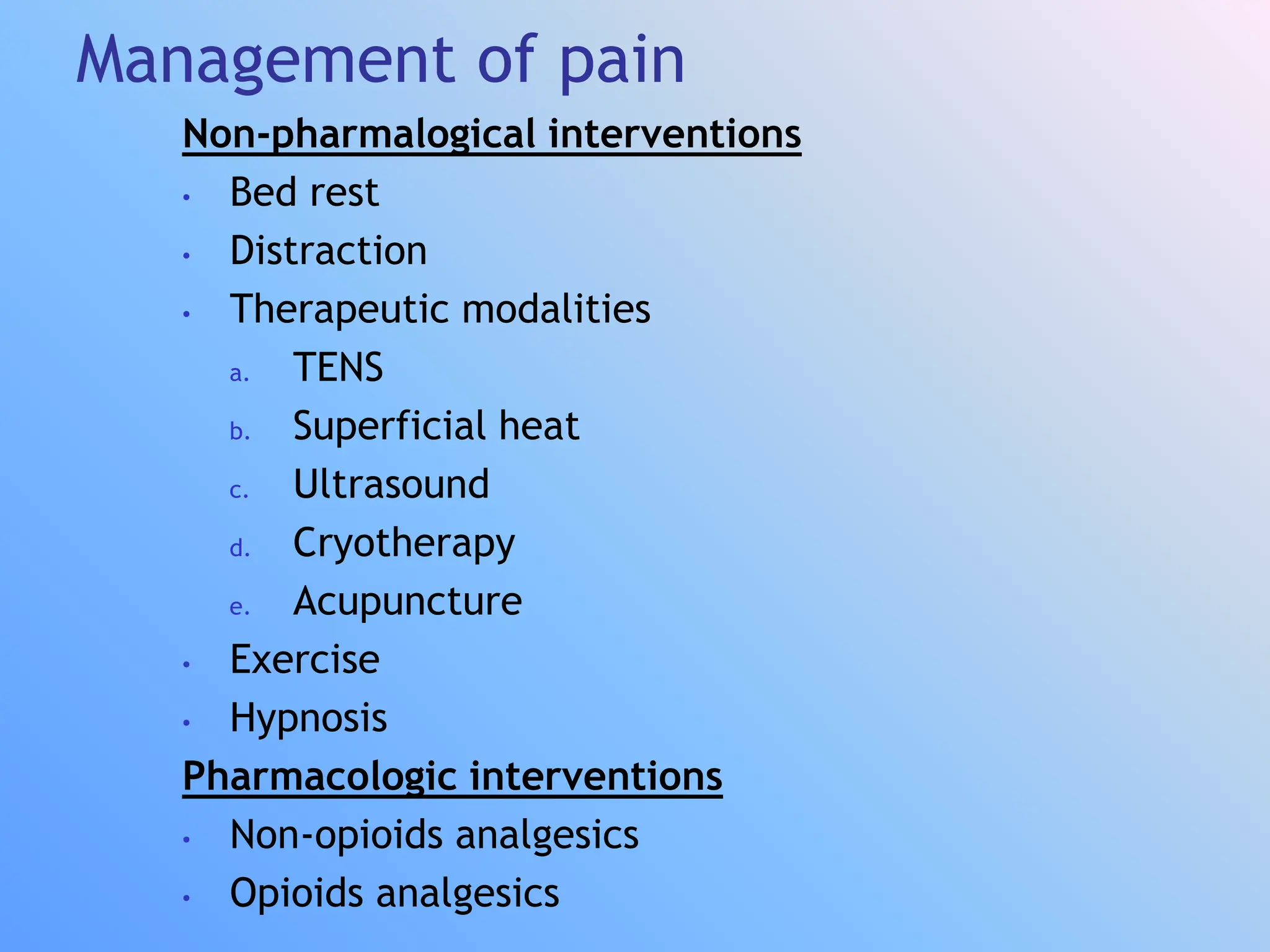
The document discusses the history, components, receptors, pathways, and management of pain. It defines pain as an unpleasant sensory and emotional experience associated with actual or potential tissue damage. Pain has fast and slow components transmitted by different fiber types to the central nervous system. Management includes non-pharmacological approaches like TENS, heat, and exercise as well as pharmacological options like non-steroidal anti-inflammatories, opioids, antidepressants, and antiepileptics. The gate control theory proposes large fibers can open or close a "gate" to modulate pain transmission in the dorsal horn.