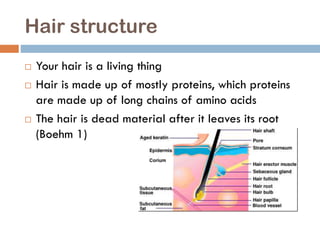
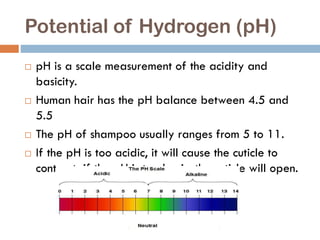
The document discusses hair structure, the function and chemistry of shampoo, and how pH impacts hair and shampoo effectiveness. Hair is made of protein chains and is dead after leaving the root. Shampoo's main ingredients are water and surfactants, which help clean hair by stripping oil. The pH of hair is 4.5-5.5, while shampoo pH ranges from 5-11; a pH that is too acidic or basic can damage hair. Shampoo aims to remove dirt while maintaining the hair's natural pH balance.